Radiologists may soon be able to calculate the optimal radiation dose for CT scans through a new technique that automatically calculates patient diameter from CT images. The protocol could eliminate the need for body mass index (BMI) calculations and manual measurements of patient size.
Radiology's renewed focus on radiation dose reduction has clinicians measuring patient size to optimize CT exposure settings to the specific patient being imaged. But measuring each patient manually is both time-consuming and inaccurate.
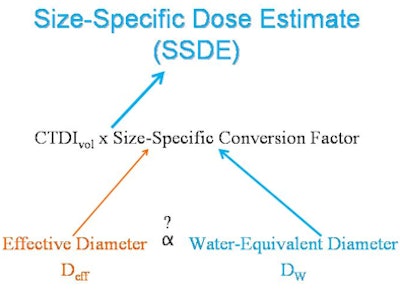
Instead, researchers from Brigham and Women's Hospital have developed a fully automated technique that permits the estimation of patient body habitus from data derived from axial CT scans, according to a presentation at the RSNA 2012 meeting. Specifically, the technique uses the size-specific dose estimate (SSDE) concept, a metric introduced in 2011 that derives measurements of organ dose based on patient size, scan length, and tissue attenuation.
In a test series of recently acquired scans, the technique yielded strong correlations with manual measurements of patient diameter, paving the way for fast and accurate SSDE calculations, the researchers said.
Patient size is essential
Determining patient size accurately is essential for adjusting radiation exposure metrics such as CT dose index volume (CTDIvol), as discussed in the 2011 Task Group 204 report published by the American Association of Physicists in Medicine (AAPM), explained Dr. Ichiro Ikuta in the RSNA presentation.
Ikuta, along with senior investigator Dr. Aaron Sodickson and colleagues, proposed an automated method to consistently and efficiently extract patient size without requiring manual measurements that are prone to variation or susceptible to irregular patient contours. The patient size measurement methodology outlined in the AAPM 204 report isn't optimal because patients aren't perfect ellipses, and they come with a wide range of different tissue attenuations, Ikuta said.
"We were looking for another surrogate for patient size," he said. "CTDIvol is a measure of x-ray tube output, not dose, and as they outlined in report 204, there needs to be a size-specific conversion factor in order to get a size-specific dose estimate."
The study included 50 CT scans of the thorax and abdomen/pelvis performed in the emergency department in January 2012; the images were reconstructed with a full field-of-view (500 mm) to visualize all skin contours. The CT images were measured using the Generalized Radiation Observation Kit (GROK), an open-source application programmed to automatically calculate the total attenuation-area product for an axial CT image based on pixel attenuation and area data. GROK outputs what the diameter of the patient would be in an equivalent body of water (Dw).
For comparison, the manual technique was used to calculate the effective diameter (Deff) of the patient from the same CT images as the automated technique.
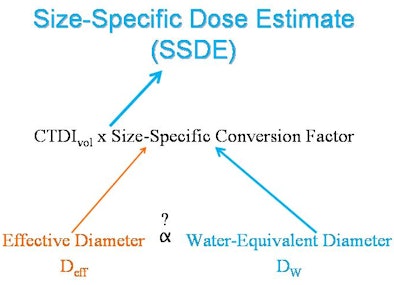 |
| CTDIvol relates to the x-rays generated by the CT scanner, rather than the x-rays absorbed by the patient. Determining the radiation dose requires a method for extracting patient size from CT to allow for more appropriate, patient-specific estimates with size-specific conversion factors. AAPM has reported these size-specific conversion factors as a function of effective diameter (Deff) calculated from manual measurements. However, manual measurements are susceptible to interobserver variability and interrupt the workflow of the radiologist. Water-equivalent diameter (Dw) is another surrogate measure of patient size, and it can be generated in an automated manner using the open-source GROK informatics toolkit. If there were a relationship between Deff and Dw, size-specific conversion factors could be automatically generated, allowing for more appropriate size-specific dose estimates without interrupting clinical workflow. Image courtesy of Dr. Ichiro Ikuta. |
"The Deff range provides an idea of only the physical dimensions of the patient, without any indication of what might be contained inside, such as high x-ray attenuating bones or low-attenuating air," Ikuta explained in an email to AuntMinnie.com. "Dw takes into account both size and x-ray attenuation of the CT beam, analogous to the CT scanner's automatic exposure control. Thus, a larger patient containing a large amount of x-ray-attenuating tissue would have a larger Dw, and the CT scanner x-ray output would also be set higher to get more of the x-rays necessary to traverse more tissue. If a part of the patient is full of air (such as the lungs), there is less tissue and a lower Dw, and also a lower CT scanner x-ray output since air does not attenuate the x-ray beam nearly as much as tissue would."
In the study, Deff was calculated as the square root of the anterior-posterior diameter multiplied by the lateral diameter per the AAPM 204 report. The water-equivalent diameter was plotted against Deff at several anatomic locations in the thorax (including the lung apex, aortic arch, carina, and diaphragmatic dome) and in the abdomen/pelvis (superior mesenteric artery, iliac crest, and L5-S1 intervertebral disk), with linear regression analysis performed separately for each anatomic region, Ikuta said.
"Based on the statistical analysis, the water-equivalent diameter was an excellent predictor of effective diameter in both anatomic locations -- a little less well-correlated in the CT of the thorax, but it still had good correlation, and there was excellent correlation of the abdomen and pelvis," he said.
For the thorax with a Deff range of 21.3 cm to 33.6 cm, R2 was 0.51 (p < 0.0001) in 200 images. For the abdomen and pelvis with a Deff range of 24.8 cm to 36.5 cm, R2 was 0.90 (p < 0.0001) in 150 axial images.
For fixed physical dimensions, variations in patient anatomy and density may produce wide variability in the associated Dw when considering the abdomen versus the thorax. For a given fixed Dw in the thorax, Deff may widely vary based upon breath-holding abilities of the patient.
"This water-equivalent diameter [technique] accounts for physical dimensions independent of patient contours, which may be irregular and introduce some error into diameter calculations," Ikuta said. "It also accounts for variations in tissue x-ray attenuation, [enabling] a new normalized scale for the attenuation area product. It correlates well with effective diameter, and it's a surrogate of patient size that can be automatically extracted from axial CT images."
After the presentation, session moderator and AAPM 204 report co-author Cynthia McCollough, PhD, from the Mayo Clinic in Rochester, MN, praised the technique as "the exact direction we need to go" to create fast and accurate patient size estimates.