Artificial intelligence (AI) software developer Avicenna.ai has garnered U.S. Food and Drug Administration (FDA) 510(k) clearance for its Cina Head AI computer-assisted triage software.
Within 20 seconds, Cina Head can automatically detect and alert radiologists to the presence of intracranial hemorrhage (ICH) and large-vessel occlusion (LVO) on CT scans of emergency room patients, according to the vendor. Radiologists are notified of these findings within their existing systems and workflow, Avicenna.ai said.
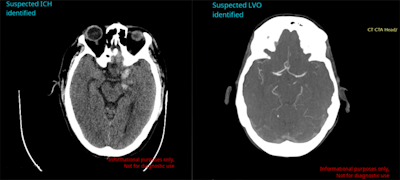 Avicenna.ai's Cina Head software can automatically detect and triage intracranial hemorrhage and large-vessel occlusion on emergency room CT scans. Image courtesy of Avicenna.ai.
Avicenna.ai's Cina Head software can automatically detect and triage intracranial hemorrhage and large-vessel occlusion on emergency room CT scans. Image courtesy of Avicenna.ai.In testing on data from 814 cases at more than 250 imaging centers in the U.S., the software yielded 91.4% sensitivity, 97.5% specificity, and 96% accuracy for detecting ICH, the company said. Cina Head delivered 97.9% sensitivity, 97.6% specificity, and 97.7% accuracy for detecting LVO on 476 cases.
Avicenna.ai said that it plans to also introduce other emergency radiology AI tools for trauma and vascular imaging applications over the next 12 months.




















