A new PET technique can visualize early Alzheimer's disease by imaging the synaptic density of the brain as indicated by the presence of a particular protein, according to a study by researchers from Yale University that was published May 13 in the Alzheimer's & Dementia Journal.
The technique uses a carbon-11-labeled radiotracer called [11C] UCB-J to image the synaptic vesicle glycoprotein 2A (SV2A) in the brain, wrote a team led by Dr. Adam Mecca, PhD. The presence of SV2A -- or lack of it -- appears linked to synaptic density, and thus, disease progression.
"Synaptic loss is a robust and consistent pathology in Alzheimer's disease and the major structural correlate of cognitive impairment," the group wrote. "PET imaging of ... SV2A has emerged as a promising biomarker of synaptic density."
Current imaging techniques for Alzheimer's disease show broad brain tissue loss or reduced brain metabolism, but [11C] UCB-J PET visualizes the distribution of synaptic damage, a more specific indication of the disease.
"Postmortem studies of Alzheimer's disease have revealed widespread synaptic loss," the group noted. "With the recent advent of synaptic PET imaging, we have begun to evaluate synaptic alterations in vivo. In our initial study of [11C] UCB-J PET in early Alzheimer's disease, we observed reduced SV2A binding restricted to the medial temporal lobe."
Mecca and colleagues used [11C] UCB-J PET to compare synapse density in 34 people with early Alzheimer's disease with 19 without it. They found high synaptic loss in areas around the hippocampus in people with early-stage Alzheimer's.
"In 34 early Alzheimer's disease compared [with] 19 cognitively normal participants, [11C] UCB-J [PET imaging] ... revealed widespread reductions of SV2A binding in medial temporal and neocortical brain regions," the group wrote. "These reductions were largely maintained after correction for volume loss and were more extensive than decreases in gray matter volume."
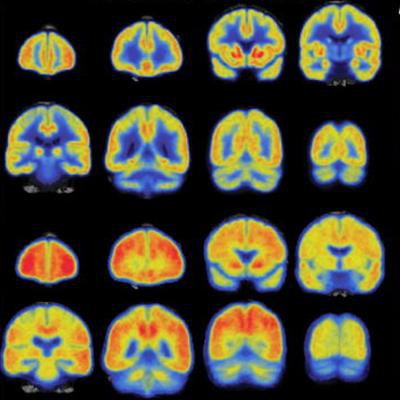
![Part A shows average synaptic density measured with [11C] UCB-J PET in a group of participants with Alzheimer's disease (right) compared with a group of participants with normal cognition (left). Widespread reduction of synaptic density is present in the group with Alzheimer's disease (AD). Part B shows average amyloid accumulation measured with [11C]PiB PET in a group of participants with AD (right) compared with a group of participants with normal cognition (left). The cognitively normal group is devoid of amyloid and the AD group has extensive amyloid accumulation. Images courtesy of Adam Mecca and Alzheimer's & Dementia: The Journal of the Alzheimer's Association.](https://img.auntminnie.com/files/base/smg/all/image/2020/05/am.2020_05_12_21_54_9896_2020_05_12_Mecca_study_image.png?auto=format%2Ccompress&fit=max&q=70&w=400) Part A shows average synaptic density measured with [11C] UCB-J PET in a group of participants with Alzheimer's disease (right) compared with a group of participants with normal cognition (left). Widespread reduction of synaptic density is present in the group with Alzheimer's disease (AD). Part B shows average amyloid accumulation measured with [11C]PiB PET in a group of participants with AD (right) compared with a group of participants with normal cognition (left). The cognitively normal group is devoid of amyloid and the AD group has extensive amyloid accumulation. Images courtesy of Adam Mecca and Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
Part A shows average synaptic density measured with [11C] UCB-J PET in a group of participants with Alzheimer's disease (right) compared with a group of participants with normal cognition (left). Widespread reduction of synaptic density is present in the group with Alzheimer's disease (AD). Part B shows average amyloid accumulation measured with [11C]PiB PET in a group of participants with AD (right) compared with a group of participants with normal cognition (left). The cognitively normal group is devoid of amyloid and the AD group has extensive amyloid accumulation. Images courtesy of Adam Mecca and Alzheimer's & Dementia: The Journal of the Alzheimer's Association.Mecca and colleagues hope their research could lead to further treatment development, as "quantification of [11C] UCB-J binding to SV2A in Alzheimer's disease may expand our understanding of Alzheimer's pathogenesis and serve as a novel biomarker for diagnosis and therapeutic efficacy," they wrote.
"Our new methods will allow us to detect widespread synaptic losses throughout the brain," Mecca said in a statement released by the university. "This gives us confidence that we may use these results as a biomarker outcome for therapeutic trials, which could help speed development of new drugs to combat the disease."