Men treated with lutetium-177 (Lu-177) prostate-specific membrane antigen (PSMA) radioligand therapy (Pluvicto, Novartis) for prostate cancer may experience neurologic problems after beginning treatment, but most are manageable through conservative methods, according to a study published August 1 in the Journal of Nuclear Medicine.
A small number of study participants did develop permanent symptoms, however, such as dysgeusia, which includes a reduced sense of taste or alterations in taste perception, according to a team led by Gokce Belge Bilgin, MD, of the Mayo Clinic in Rochester, MN.
“As with any novel treatment, understanding the full spectrum of its long-term impact in real-world settings is crucial for optimizing patient care,” Bilgin and colleagues wrote.
Lu-177 PSMA radioligand therapy (Pluvicto, Novartis) was approved for the treatment of patients with metastatic prostate cancer in March 2022. Data from clinical trials indicate that the drug is effective and safe, yet since the treatment is likely to be used in patients with a longer life expectancy, identifying potential toxicities that might affect patients' quality of life is increasingly critical, the authors noted.
To date, data examining potential neurologic symptoms after Lu-177 PSMA-617 treatment are scarce, they added. To address this knowledge gap, the group reviewed clinical records of 185 patients who received their initial dose of Lu-177 PSMA-617 at the Mayo Clinic Rochester between March 2022 and November 2022. Patients were a median age of 70 years old (range, 58 to 90) and were followed up until January 2024.
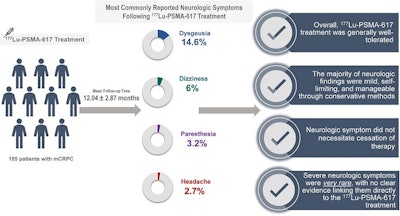 A graphical abstract of the study.Image courtesy of the Journal of Nuclear Medicine
A graphical abstract of the study.Image courtesy of the Journal of Nuclear Medicine
According to the findings, 55 new or worsening neurologic symptoms were observed in 50 patients (27%, 50/185), as follows:
- 27 patients (14.6%) reported predominantly permanent alterations in taste perception, such as a perception of bitterness, metallic taste, or spiciness, compared with their taste perception before undergoing therapy. All of these patients also reported experiencing dry mouth in addition to dysgeusia, the researchers noted.
- 11 patients (6%) experienced mild dizziness without any other clear underlying cause.
- Six patients (3.2%) reported paresthesia symptoms (tingling sensations or numbness).
- Five patients (2.7%) experienced headaches during or immediately after the treatment.
- Two patients (1.1%) presented with nondisabling, mild weakness in their extremities.
- One patient (0.5%) exhibited gait disturbances.
- One patient (0.5%), experienced a vision disturbance 5.6 months after initiating therapy.
“None of the patients discontinued or failed to complete the Lu-177 PSMA-617 therapy because of neurologic symptoms,” Bilgin and colleagues reported.
The prevalence and severity of neurologic symptoms could vary within a larger and more diverse patient population and the follow-up period and population size in this study might not have been sufficient to detect delayed or uncommon neurologic symptoms, the researchers wrote.
Nonetheless, Lu-177 PSMA-617 treatment was generally well-tolerated, with most neurologic findings being mild, self-limiting, or manageable through conservative methods.
“In patients without neurologic symptoms or central nervous system metastases before treatment, we found the development of severe neurologic problems to be rare and unlikely to require discontinuation of treatment,” the researchers concluded.
The full study is available here.