A team at the University of Pennsylvania has shown for the first time how the opioid overdose treatment naloxone blocks mu-opioid receptors throughout the whole body.
The study employed the group’s prototype long axial field-of-view scanner PennPET Explorer and reveals differences among men and women in how naloxone works, noted lead author Jacob Dubroff, MD, and colleagues.
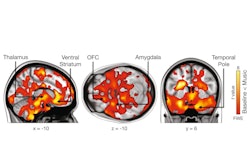
“Greater [mu-opioid receptor] occupancy in the thalamus, amygdala, and hippocampus by the MOR antagonist naloxone in women than men suggests that sex differences could affect the efficacy of opioid overdose treatment,” the group wrote. The study was published May 8 in the Journal of Nuclear Medicine.
The mu-opioid receptor (MOR) is the target of opioid drugs, including fentanyl and heroin, as well as methadone and buprenorphine, which are used to treat opioid addiction. Dubroff and colleagues are involved in research to better understand the interactions of agonists and antagonists with MORs to elucidate the etiology, prevention, and treatment of opioid use disorder (OUD).
In a previous study using PennPET Explorer, the group visualized different brain activity in people taking methadone or buprenorphine for OUD. In this study, the team conducted the first human study using the scanner to investigate the distribution of MORS in the whole body.
The investigators recruited six healthy women and seven healthy men for the study and performed imaging scans with carbon-11 (C-11) radiotracer, which binds to MORs. Participants underwent two scans, one at baseline and one immediately after pretreatment with naloxone.
 A visual abstract of the study.Journal of Nuclear Medicine
A visual abstract of the study.Journal of Nuclear Medicine
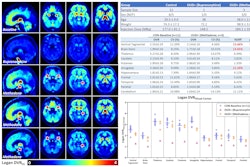
According to the findings, naloxone reduced MOR availability by 40% to 50% in the caudate, putamen, thalamus, amygdala, and ventral tegmentum, brain regions known to express high levels of MORs. Women showed greater receptor occupancy in the thalamus, amygdala, hippocampus, and frontal and temporal lobes and a greater reduction in thalamic MOR availability than men (p < 0.05).
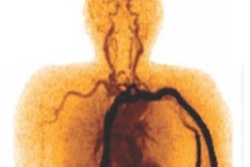
In addition, the imaging showed robust differences in C-11 carfentanil signal between baseline and naxalone scans in cervical spinal bone marrow (p = 0.002), the cervical spinal cord (p = 0.0004), and thoracic spinal cord (p = 0.025).
“Here, we present the first human whole-body neuroreceptor PET imaging study using C-11 carfentanil in which extra-central nervous system reference regions were investigated,” the group wrote.
Ultimately, the study underscores the potential clinical relevance of MOR-related sex differences, the group wrote. Although dynamic interactions of naloxone with MORs have been studied with C-11 carfentanil PET in men, the researchers noted that sex differences in naloxone binding have previously not been examined.
“Sex differences in naloxone-MOR interactions should be further investigated to provide additional insights into opioid neuropharmacology, especially given the present opioid epidemic,” the group concluded.
The full study is available here.