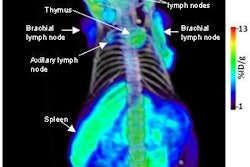
Molecular Insight Pharmaceuticals of Cambridge, MA, has begun a phase I clinical trial for its Trofex molecular imaging program for detecting and staging metastatic prostate cancer.
The trial is designed to investigate two small molecule radiopharmaceutical candidates that target prostate-specific membrane antigen (PSMA), MIP-1072 and MIP-1095, to select a lead candidate for further development and commercialization.
The company is conducting the study under an exploratory investigational new drug application submitted to the U.S. Food and Drug Administration.
The trial will study as many as 12 patients, who will receive a single dose of MIP-1072 or MIP-1095, followed by a single dose of the alternate candidate compound 14 days later. The study is being conducted at Duke University Medical Center in Durham, NC; New York Presbyterian Hospital-Cornell Medical Center in New York City; and Johns Hopkins Hospital in Baltimore, MD.
Related Reading
Molecular Insight's revenue decreases in Q1, May 14, 2008
Molecular Insight launches new Azedra trials, May 1, 2008
Molecular Insight raises $150 million, November 12, 2007
Molecular Insight completes phase I Azedra trial, October 18, 2007
Molecular Insight buys manufacturing site, October 3, 2007
Copyright © 2008 AuntMinnie.com