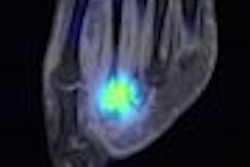
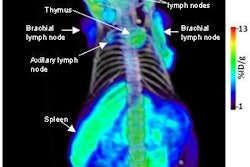
Lantheus Medical Imaging of North Billerica, MA, reported results from a phase I clinical trial of a new cardiac PET radiopharmaceutical at this week's SNM meeting in New Orleans.
The company is researching BMS747158, a fluorine-18-based PET agent for myocardial perfusion. The firm said that data from the safety and tolerability study of the agent presented at the SNM show indicate that BMS747158 has a "favorable radiation dosimetry profile and is generally well-tolerated." In addition, the research showed "high myocardial uptake that was stable over time with favorable myocardial to background ratios."
Lantheus believes that BMS747158 could serve as a new class of PET agents for myocardial perfusion imaging. The radiopharmaceutical has "high, rapid, and sustained cardiac uptake proportional to blood flow, slower washout, higher target to nontarget uptake ratios, ability to image perfusion deficits, and capability to produce high quality of images taken in multiple species compared to currently marketed tracers," the company said.
The research presented this week was conducted by Dr. Jamshid Maddahi of the David Geffen School of Medicine at the University of California, Los Angeles.
Related Reading
Lantheus updates Definity label, May 13, 2008
Lantheus launches new Definity trial, May 7, 2008
Lantheus focuses on cardiac imaging following BMS spinoff, March 27, 2008
Bristol-Myers Squibb unit becomes Lantheus Medical Imaging, March 18, 2008
BMS posts last financials for medical imaging business, February 1, 2008
Copyright © 2008 AuntMinnie.com