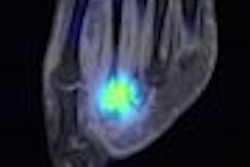
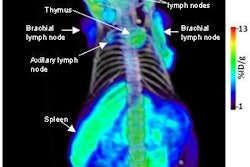
Radiopharmaceutical developer Molecular Insight Pharmaceuticals of Cambridge, MA, is touting the results of three clinical studies of its Trofex prostate cancer detection agent that were presented at this week's SNM meeting in New Orleans.
The presentations described the synthesis, production, and preclinical profile of two compounds, MIP-1072 and MIP-1095, that Molecular Insight is researching with the goal of selecting one of them for further commercial development.
Both compounds target prostate-specific membrane antigen (PSMA), a protein that is highly expressed by prostate tumor cells. Molecular Insight hopes Trofex will enable clinicians to identify and stage tumors, particularly in metastatic disease in which patients have no obvious symptoms other than increasing levels of established biomarkers such as prostate-specific antigen levels.
Related Reading
Molecular Insight starts Trofex trial, June 12, 2008
Molecular Insight's revenue decreases in Q1, May 14, 2008
Molecular Insight launches new Azedra trials, May 1, 2008
Molecular Insight raises $150 million, November 12, 2007
Molecular Insight completes phase I Azedra trial, October 18, 2007
Copyright © 2008 AuntMinnie.com