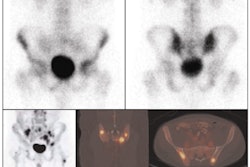
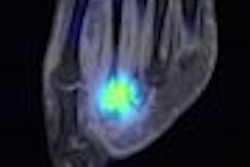
Gamma camera probe developer Neoprobe of Dublin, OH, has inked a letter of intent to collaborate with DraxImage, the company said.
According to the nonbinding agreement, Kirkland, Quebec-based DraxImage will exclusively market and distribute Neoprobe's Lymphoseek product in the European Union, Switzerland, Scandinavia, Turkey, Canada, and India. Unradiolabeled Lymphoseek kits will be marketed and sold to hospitals through DraxImage's marketing channels in these countries.
Neoprobe has completed a multicenter phase II clinical study for Lymphoseek that evaluated the efficacy and safety of the agent in patients with melanoma or breast cancer. The company has started enrolling patients for the first of two phase III clinical trials that will assess the safety and efficacy of Lymphoseek as a sentinel node tracing agent.
Related Reading
Neoprobe launches new gamma detection system, June 25, 2008
Neoprobe starts phase III trial, June 9, 2008
Neoprobe gets OK for Lymphoseek study, April 16, 2008
Neoprobe announces phase II Lymphoseek results, March 13, 2008
Neoprobe nets financing, December 27, 2007
Copyright © 2008 AuntMinnie.com