GE Healthcare is touting promising results from a phase I clinical trial of GE-067, a fluorine-18-labeled PET imaging agent being developed by the Chalfont St. Giles, U.K.-based company to assist in the detection of brain beta-amyloid.
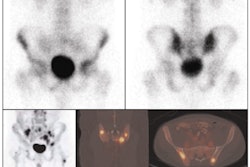
The study shows greater brain uptake of GE-067 in Alzheimer's disease patients compared with healthy volunteers. The two-hour half-life of the fluorine-18-labeled ligand is designed to offer the potential for greater clinical accessibility compared with the research compound [11C] PIB.
The phase I study consisted of 22 subjects who were injected with GE-067. Initially, six healthy volunteers had whole-body scans to determine radiation dose. In addition, eight healthy and eight probable Alzheimer's subjects had cranial scans with significant amounts of uptake observed in the probable Alzheimer's brains compared to the healthy volunteers.
The initial results suggest that GE-067 could be used to detect amyloid pathology in vivo and potentially contribute to future diagnostic and treatment algorithms, subject to performance of confirmatory studies.
Rik Vandenberghe, Ph.D., of the University of Leuven in Belgium presented the results this week at the International Conference on Alzheimer's Disease in Chicago.
Related Reading
GE consolidates into four business segments, July 28, 2008
GE to buy monitor firm Vital Signs, July 24, 2008
GE moves Optison agent through EU regulation, July 23, 2008
Dineen replaces Hogan at GE Healthcare, July 17, 2008
GE adds charges for MRI scanners, July 15, 2008
Copyright © 2008 AuntMinnie.com