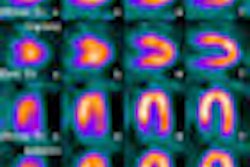
Nuclear medicine vendor Digirad has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its nSpeed reconstruction software.
nSpeed is designed to improve image quality and reduce imaging time for SPECT procedures, according to the Poway, CA-based firm.
Related Reading
Trial begins for Digirad's Cardius X-ACT, September 5, 2008
Novel cardiac SPECT system improves attenuation correction, August 15, 2008
Digirad posts loss in Q2, July 29, 2008
Digirad signs deal with U. of Chicago, April 29, 2008
Digirad Q1 results dip into red, April 24, 2008
Copyright © 2008 AuntMinnie.com