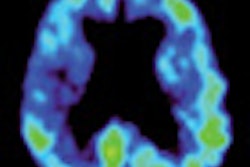
A panel of medical experts from the U.S. Food and Drug Administration (FDA) says imaging agents currently in development could help doctors diagnose abnormal amyloid plaques in the brain associated with Alzheimer's disease.
The Wall Street Journal quotes Dr. Rafel Rieves, director of the FDA's office of medical imaging, as saying the agency wants to craft guidelines for companies to conduct clinical trials for the possible clearance and commercial use of the agents. He added that the agents currently are a few years away because no proposed studies have begun.
The comments came yesterday following an FDA meeting of an outside panel of medical experts to provide advice on how such studies should be designed.
The imaging agents would be used with PET to detect areas of the brain that contain the beta amyloid protein, which is present in plaques in the brains of people who have died of Alzheimer's complications.
Bayer Schering Pharma of Berlin; GE Healthcare of Chalfont St. Giles, U.K.; and Avid Radiopharmaceuticals of Philadelphia are developing imaging agents designed to detect amyloid via PET imaging.
Related Reading
PET with C-11 PiB can assess beta-amyloid brain deposits, August 12, 2008
PET scans help spot and classify dementia types, March 27, 2008
FDG-PET CAD scheme boosts diagnostic accuracy for Alzheimer's, October 2, 2007
FDG-PET can influence Alzheimer's, dementia treatment, September 6, 2007
PET shows promise in atypical dementia evaluation, August 29, 2007
Copyright © 2008 AuntMinnie.com