Nuclear medicine firm Digirad has received U.S. Food and Drug Administration (FDA) 510(k) clearance for new applications on its ergo imaging general-purpose nuclear medicine camera.
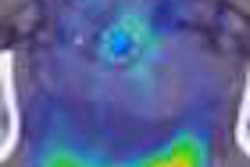
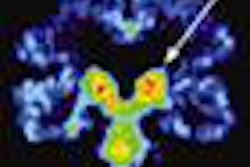
The clearance expands the system's clinical flexibility in areas such as nuclear medicine, surgery, and women's health, according to the vendor. It includes indications for lymphatic scintigraphy and parathyroid scintigraphy, as well as for aiding the evaluation of lesions in the breast and other small body parts, Digirad said.
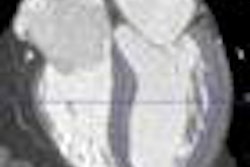
The system can also be used intraoperatively when protected by sterile drapes, the company said. When used for breast imaging, ergo is now indicated to serve as an adjunct to mammography or other primary breast imaging modalities.