CT has carved out a useful niche in the early assessment of stroke victims, helping determine who receives brain-saving therapy with recombinant tissue plasminogen activator (tPA) drugs. But MRI may ultimately prove more versatile than CT for early stroke assessment, and hospitals may someday choose to site MRI scanners closer to emergency departments to take advantage of the crucial window during which the brain can be saved through early tPA thrombolytic intervention.
That’s the assessment of MRI expert Dr. William Bradley, who spoke on the topic of MRI in acute stroke at the 2004 Indian Radiological and Imaging Association (IRIA) meeting in January in Hyderabad. Bradley is the chairman of the radiology department at the University of California, San Diego in the U.S.
Stroke affects some 600,000 people in the U.S. and is the third leading cause of death in the country, Bradley said. Stroke victims who arrive at the emergency department early enough can be treated with tPA, which can preserve brain function if administered intravenously within three hours of stroke onset (or six hours if given intra-arterially).
But tPA should be used judiciously. Some patients arrive at the emergency department with no brain tissue that can be saved by thrombolytic therapy, while others suffer from cerebral hemorrhages that would be aggravated by tPA administration.
CT can help predict which patients are at risk of hemorrhage, and as a result many hospitals have started placing scanners next to ER units. But CT has drawbacks -- it is incapable of diagnosing stroke at present; this leads to overuse of tPA, with 60% of the patients who don’t need it receiving the drug, according to Bradley. Some 20% of cases are misdiagnosed as stroke, 20% are cases of multiple stroke in which tPA isn’t appropriate, and 20% are cases in which there is no brain to save.
MRI diffusion and perfusion imaging may prove to be a more accurate way to determine who should receive thrombolytic therapy, he said.
“CT isn’t used to confirm the diagnosis of stroke, it is just used to exclude hemorrhage,” Bradley said. “There is actually very little that radiologists have to offer in the treatment and triage of stroke patients for thrombolysis. That will change. In the next 5-10 years you will see MR used more and more to triage the appropriate use of thrombolytics.”
For example, T2-weighted fluid-attenuation inversion recovery (FLAIR) MRI sequences can be used to diagnose parenchymal and subarachnoid hemorrhage, Bradley said, enabling clinicians to exclude these patients from tPA therapy. The key is to look for the deoxyhemoglobin border sign, a dark, irregular border that appears within minutes and that can be easily confused with a hemosiderin rim in chronic hemorrhage cases. This sign represents deoxyhemoglobin formation at the interface between the actively metabolizing brain and the outer portion of the hematoma.
“If we are going to be using MRI to triage stroke patients you absolutely need to look for this sign,” Bradley said. “After I heard of this sign I went back and looked at several hundred hyperacute hematoma (cases) I had been collecting, and they all had this sign.”
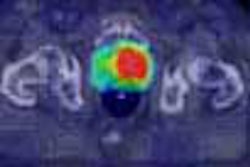
With FLAIR, MRI is now even more sensitive than CT in diagnosing hyperacute parenchymal hematomas, giving clinicians more confidence in excluding hemorrhage cases from receiving tPA. But thanks to new echo-planar imaging (EPI) sequences that enable diffusion and perfusion imaging, MRI gains even more versatility than CT, Bradley said.
An EPI diffusion scan can confirm the diagnosis of stroke, thanks to the light bulb sign -- an area of bright signal -- caused by the different appearances on MRI between normal water and water that has permeated cells as cytotoxic edema. Normal extracellular water experiences dephasing and a loss of signal, and thus appears dark on diffusion EPI. But water trapped inside cells as cytotoxic edema doesn’t move out of phase in the presence of strong gradients, and as a result remains bright, Bradley said.
Diffusion imaging is also useful for diagnosing acute right hemiparesis. Acute infarcts become bright with diffusion imaging almost immediately, and then stabilize and become iso-intense three to four weeks later. After that, infarcts appear darker than normal cerebrospinal fluid (CSF) in the area where the infarct occurred.
Diffusion imaging does have some limitations. For example, the symptoms of cytotoxic edema that create the light bulb sign on diffusion MRI do not occur until blood flow in the brain experiences an 80% drop, to 10 ml/100 g/minute, compared to normal blood flow of 50 ml/100 g/min. However, loss of function and EEG abnormalities occur at 17 ml/100 g/min, a 66% drop from normal, leaving a window in which a patient could be having a stroke before the symptoms would show positive on a diffusion scan.
EPI perfusion is capable of picking up these patients, Bradley said. The technique involves the injection of a bolus of gadolinium, which is paramagnetic and causes T2* shortening on the gradient-echo image. Approximately 40 images are acquired in a 128 x 128-pixel matrix, and computer post-processing produces perfusion maps that track the mean transit time (MTT) of the contrast bolus through the patient’s capillaries, and the relative cerebral blood volume (rCBV).
Dividing rCBV by MTT produces the relative cerebral blood flow (rCBF), a crucial measure in determining which patients will bleed flowing tPA administration. Comparing rCBF in the infarcted hemisphere to the contralateral, normal hemisphere will produce a critical ratio: if the ratio of rCBF in the infarcted hemisphere is less than 35% of the normal side, then the patient will bleed. If the infarcted rCBF is greater than 55%, no bleeding would occur up to 12 hours later, according to published research.
“We are beginning to hone in on the fact that low blood flow to the brain correlates with an increased tendency to bleed, either through natural thrombolysis of the clot or assisted with a thrombolytic agent like tPA,” Bradley said.
Perfusion maps are also valuable when compared to diffusion images. The diffusion image shows the core of the infarct, while the perfusion maps demonstrate the area that’s at risk of further cell death if therapy is not given -- an area known as the ischemic penumbra. In this zone, rCBF is in the range of 17-20 ml/100 g/min, and while the neurons are not functional they can be salvaged if blood flow can be increased through thrombolysis.
Bradley concluded by calling for clinical trials comparing MRI with CT to demonstrate its effectiveness in stroke assessment. If MRI’s clinical utility is proven, employing FLAIR will enable radiologists to exclude hemorrhage, as CT does currently, while EPI diffusion/perfusion will enable treatment to be more customized for patients.
But the speed is a major caveat, which is the reason why MRI scanners must be sited next to the ER in order to perform stroke assessment effectively.
“It’s the old axiom time is brain. You have to be able to do it very quickly and do it in under ten minutes,” Bradley said. “We have a seven-minute stroke study, which gives us a minute to two to get the patient on and off the table.”
By Brian Casey
AuntMinnie.com staff writer
March 12, 2004
Related Reading
Perfusion CT predicts, measures, thrombolysis benefit after acute stroke, December 24, 2003
Drinking associated with brain shrinkage, December 5, 2003
Three-minute MRI exam deemed feasible, beneficial, December 2, 2003
New approaches aid CT perfusion for ischemia, May 23, 2003
Copyright © 2004 AuntMinnie.com