Guerbet has received a unanimous recommendation from the U.S. Food and Drug Administration's (FDA) Medical Imaging Drugs Advisory Committee that the agency approve the company's new drug application (NDA) for MRI contrast agent Dotarem (gadoterate meglumine) for use in adults and children 2 years and older.
The committee voted 10 to 6 -- with one member abstaining -- not to recommend approval at this time for children younger than 2 years of age, the firm said.
The committee's recommendations are not binding, but the FDA does consider the panel's recommendation in the final assessment of the NDA.
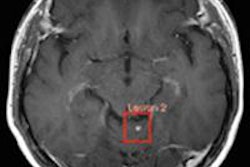
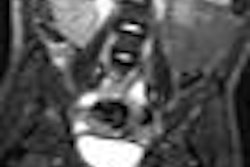
Dotarem is a gadolinium-based contrast agent for intravenous use with MRI in the brain (intracranial), spine, and associated tissues in adults and pediatric patients to detect and visualize areas with disruption of the blood-brain barrier and/or abnormal vascularity. The recommended dose is 0.1 mmol Gd/kg, Guerbet said.
Dotarem data presented at the February 14 advisory committee meeting included results from two phase III clinical studies to evaluate the diagnostic efficacy of Dotarem in MRI of diseases of the central nervous system, such as primary or secondary tumors of the brain or spinal cord, inflammatory diseases such as multiple sclerosis, and vascular brain diseases.