The U.S. Food and Drug Administration (FDA) has cleared MRI technology firm SpinTech MRI's postprocessing software, STAGE (Strategically Acquired Gradient Echo).
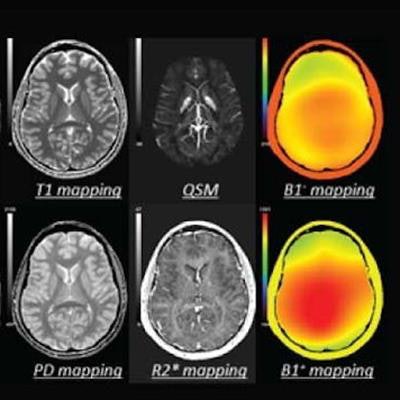
The software allows users to acquire 16 brain contrast images -- including 10 enhanced contrast qualitative outputs and six quantitative outputs -- in five minutes on 3-tesla systems, the company said.
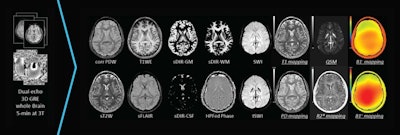 SpinTech's STAGE software is designed for postprocessing of brain images. Image courtesy of SpinTech.
SpinTech's STAGE software is designed for postprocessing of brain images. Image courtesy of SpinTech.STAGE is compatible with most MRI systems, according to the firm, and it is in use at more than 50 hospitals, imaging centers, and research facilities around the world.




















