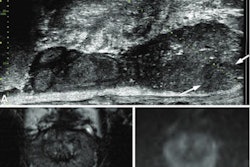
Smart Soft Healthcare of Bulgaria has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for CoLumbo, an image postprocessing and measurement application that provides quantitative measurements from MR images of the lumbar spine.
CoLumbo is designed to support review, analysis, and interpretation of lumbar MR images. It includes functionality that supports feature segmentation, feature measurement, threshold-based labeling of out-of-range measurement, and export of measurement results to a written report.
Smart Soft noted that the FDA clearance is an important milestone in the company's planned expansion into the U.S. market. In Europe, CoLumbo has been tested in three European hospitals and recently received the CE Mark.