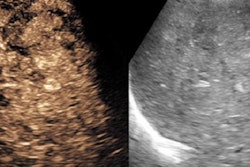
A laboratory at the University of California, Davis, is finding new ways to harness ultrasound for the exploration of disease processes, aiming to move its experimental models into clinical practice as rapidly as possible. Heading the effort is Katherine Ferrara, Ph.D., a researcher with great faith in tiny bubbles.
"I'm a real believer that ultrasound has the potential to be translated into the clinic for a number of these kinds of studies," Ferrara said at the 2007 American College of Radiology Imaging Network (ACRIN) fall meeting in Washington, DC. "As you know it's inexpensive, fast, safe, (and) nonionizing, and it can be used in real-time to guide many interventions."
The purely intravascular contrast agents tested at the university's Center for Molecular and Genomic Imaging are based on microspheres -- tiny gas bubbles typically filled with a perfluorocarbon, she said. The outer shell is made of lipid, or alternatively of albumen, or a polymer.
Ferrara talked about the group's experiments with microspheres aimed at finding new functional imaging applications using multiple imaging modalities.
"Historically, a lot of what we've done is take little pictures of the bubbles as they expand," Ferrara said. "As a bubble is hit by an ultrasound wave -- this is at 1 MHz at fairly strong pressure -- it can be (made) multiple times larger than it was at rest. So it's the expansion and contraction of this little gas bubble that allow us to hear even an individual bubble as it moves around in the circulatory system. Also, if you drive it very hard, it breaks up into little tiny pieces, which sound different from the original bubble."
The activity of the microspheres generates two principal sources of image contrast: one, the "exuberant" behavior of the bubbles in surrounding tissues; and two, the bubbles' ability to be broken up by sound waves, which enables the researchers to probe the differences in sound waves returned during and after their destruction, Ferrara explained.
"If we send out a certain pulse, we get (back) energy at the same frequency (and) energy at higher frequencies, and the sum of those two contains components that allow us to recognize where a bubble is," she said.
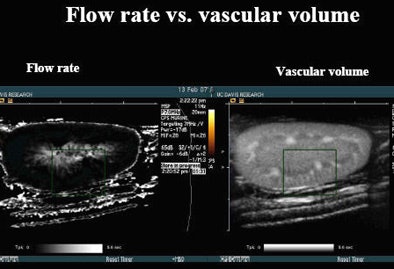 |
| In laboratory experiments, microbubble contrast agents are monitored as they flow into tumor tissues. When ultrasound waves are used to create a destructive event that washes out contrast, reperfusion of an organ can be monitored. The time to reperfusion of contrast versus the flow rate measures the number of seconds it takes to refill a rat kidney with a microbubble contrast agent after it has been destroyed with sound waves. The image indicates that the central medulla is brighter than the surrounding cortex, which refills more quickly. The same data can be used to map vascular volumes. All images and data courtesy of Katherine Ferrara, Ph.D. |
The group is accumulating quantitative measurement models that could potentially be translated into clinical practice -- for example, in monitoring the response to a new drug, or developing operator-independent techniques to assess organ function.
Among the most promising models is "time to reperfusion," which measures how long it takes for a region to return to a certain fraction of its original level after the microsphere contrast agent has been disrupted, Ferrara said.
The focus on reperfusion is not because this parameter represents an ideal physiologic target for functional measurement, but rather because "it's a really good parameter for ultrasound -- in the sense that if we can find measurements that are a fraction of current levels, or ... ratios of parameters, then they're attenuation-independent and we can use them in a range of organ systems in a very repeatable, quantitative way," Ferrara said.
Other experiments have measured areas of the curve at ultrasound and compared them to measurements from other imaging modalities. But time to reperfusion and other ratio-based measures work best, she said. Kidneys, specifically rat kidneys, have been the animal model of choice for determining what can be easily quantified.
The number of seconds needed to reperfuse the kidney after the contrast has been destroyed by a sound wave can be quantified, and the same data used to make vascular volume maps, Ferrara said. Real-time motion correction has advanced the approach substantially because it enables the refilling to be viewed in the context of major vasculature, and measured far more accurately than uncorrected data.
With sufficient contrast material and the increased accuracy that comes with motion correction, contrast inflow into the organ can be mapped over time, with yellow regions, for example, representing the fastest flow rate. And research into nonlinear encoding of flow rates is improving the researchers' ability to color code velocities.
Using drugs such as hydralazine or dopamine to perturb the flow within systems and examine the resulting changes enables further understanding of flow dynamics.
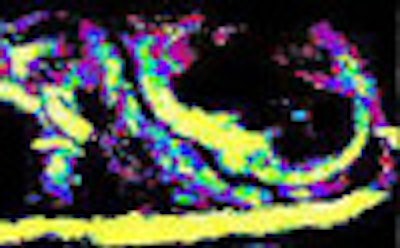
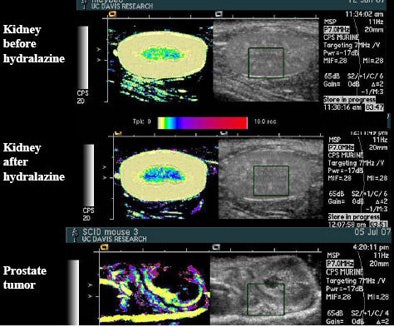 |
| Drugs such as hydralazine and dopamine can be used to perturb the flow of microbubble contrast agents to improve the depiction of perfusion in an organ. The main change seen after administration of the drug hydralazine in the rat kidney, above, was that flow rate slowed, represented by a darker blue in the medulla. Experiments with the flow-perturbing drugs have been shown to be easily reproducible. |
"We can quantify the increase in the amount of time we slow it down by using a drug like dopamine, and if we make multiple measurements of the same region, our standard deviations are small," she said. "These are really good, repeatable studies for this kind of technique." And the ultrasound results can be compared to MRI.
The group has applied this technique to cancer, specifically the refill of displaced contrast in a transgenic breast tumor. Using a 20-second nonlinear map, the tumor can be seen in about eight seconds, Ferrara said.
From single imaging studies, the team has moved on to multimodality studies that measure drug response via perfusion changes over time. The process has been improved by motion correction and the development of 3D field-of-view, enabling real-time quantitation, she said.
US plus molecular
Further away from clinical implementation is molecular imaging, with FDG-PET-labeled radiopharmaceuticals, as well as more advanced agents.
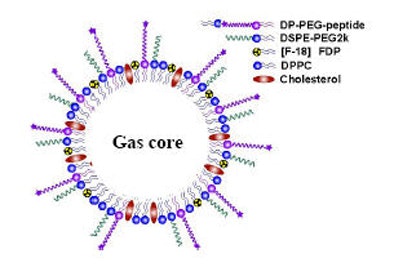 |
| Multiple targeted molecular agents within microspheres can be directed by ultrasound waves to potentially illuminate any number of pathologic processes. |
In one experiment, the group made a radioactive lipid and put it in a microsphere delivery vehicle to acquire dual measurements of where the microbubbles go with both PET and ultrasound, Ferrara said. Vascular-targeted agents can be used in this way to measure contrast accumulation.
"You can make these sorts of measurements on any clinical ultrasound system," Ferrara said. Injecting the agent over about six minutes, "you would see the agent come in, and there would be some circulating agent that would die off over time," she said. "There would also be some agent that becomes bound to your region of interest, and you would be tracking some total signal that changes over time, which includes the combination of the targeted agent and the circulating agent."
Once both agents have been tracked, the application of a destructive pulse sequence can be used to bring the measurements back to baseline. Comparing images acquired before and after the destructive burst can be used to estimate how much bound contrast is located in the region of interest, Ferrara said.
The preburst/postburst model can be used to compare two different imaging modalities or two different techniques, such as a targeted agent and nontargeted agent. With a frame of reference as to how much contrast is expected to accumulate, it is possible to get a good sense of whether angiogenesis is present. And many other potential models are ripe for development, Ferrara said.
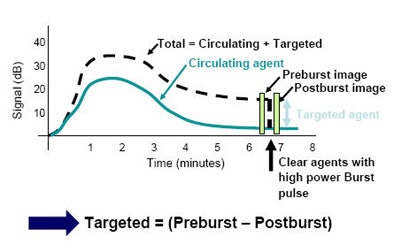 |
| Measuring contrast accumulation preburst and postburst -- before and after destruction with sound waves -- can be used to quantify the accumulation of targeted agents, and therefore angiogenesis in the region of interest. |
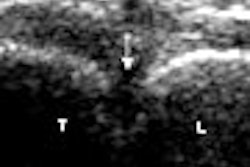
"The other neat toy we have is that we can actually press our agents against the vessel wall," she said. "We can use the force of the ultrasound wave to cause the gas bubbles to expand and contract, but the momentum of the wave deflects them to the far vessel wall. And this happens at very low ultrasound pressures -- well below pressures you would typically use to make an ultrasound image." Altering the pressures over a range can be used to modify the signal.
Another big opportunity is in real-time molecular imaging, which will permit ultrasound assessment of multiple targeted regions in a very quick exam. It is even possible to "push" bound and targeted agents simultaneously with ultrasound waves to multiple sites.
"We can do this with a very high contrast-to-free-bubble ratio, so we can get very good differentiation of the bound agent from the free agent," Ferrara said.
"I think ultrasound has the potential for quantitative turnkey real-time imaging -- something like what is commonly done for CT and MRI for specific targets," she said. "We're looking at breast, prostate, and bladder cancer right now in my group -- direct comparisons with CT and MRI to follow the effects of anti-angiogenic drugs as a proof of concept. And I think this is an area where ultrasound has real potential."
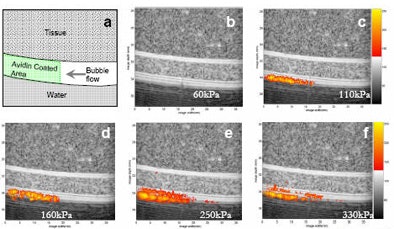 |
| Ultrasound images of a tissue and vessel phantom (grayscale) containing targeted contrast agents (orange). In this in vitro study, targeted contrast agents containing biotin in the shell flow through a vessel coated by avidin. The targeted agents are forced to the vessel wall by ultrasound pressure, but bind only where the "receptor" (avidin) is present. Once the contrast agents bind, an image of their location is created using ultrasound pressure ranging from 60 to 330 kPa. A clear image of the location of the bound contrast agents can be created. |
Several research teams are developing agents that could be ready for human trials within the next year or two. But while real-time targeted molecular imaging is a great thing to have on the radar, finding the right application will be critical, Ferrara said.
By Eric Barnes
AuntMinnie.com staff writer
February 12, 2008
Related Reading
Ultrasound contrast advocates take aim at FDA black box warning, January 3, 2008
MRgFUS an option for many women seeking less invasive fibroid treatment, June 18, 2007
ASE unveils new stress echo guidelines, September 7, 2007
Contrast media add valuable information in US applications, May 4, 2007
Echo functionality grows with processing power, August, 31, 2006
Copyright © 2008 AuntMinnie.com