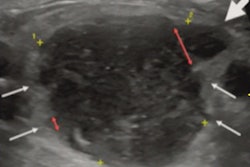
Breast ultrasound (US) technology developer iSono Health has received U.S. Food and Drug Administration (FDA) clearance for ATUSA, an automated, wearable whole-breast ultrasound system.
Designed to make 3D breast ultrasound available at the point of care, ATUSA includes a wearable accessory and artificial intelligence (AI)-based software for automated image acquisition and analysis, according to the vendor. The portable system can automatically scan the entire breast volume in two minutes and yield 3D visualization of the breast tissue, iSono Health said.
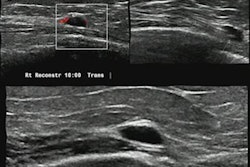
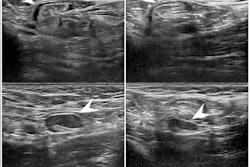
 iSono Health's ATUSA automated whole-breast ultrasound system features a wearable patient accessory. Image courtesy of iSono Health.
iSono Health's ATUSA automated whole-breast ultrasound system features a wearable patient accessory. Image courtesy of iSono Health.What's more, it can integrate with AI models to provide physicians with tools to assist in decision-making and patient management, according to the company. iSono Health said it's planning several other submissions to the FDA. Prospective case collection studies are being conducted to validate various deep-learning models to assist in localizing and classifying breast lesions, according to the vendor.