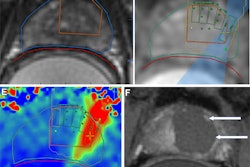
MRI-guided focused ultrasound (MRgFUS) combined with chemotherapy boosted overall survival for patients with glioblastoma in a study published November 24 in The Lancet Oncology.
Researchers led by Graeme Woodworth, MD, from the University of Maryland Medical Center in Baltimore found that this approach led to a nearly 40% increase in overall survival in a cohort of 34 patients, something the researchers are calling a first.
“The results… suggest that adding [MRgFUS] to monthly upfront adjuvant standard-of-care temozolomide is a promising combination therapeutic approach for individuals with high-grade glioma,” Woodworth and colleagues wrote.
Glioblastoma is the most common and deadliest type of malignant brain tumor with a five-year survival rate of 5.5%. Chemotherapy is typically used to treat these tumors, but most treatment drug therapies are blocked by the blood-brain barrier. This thin membrane of vascular and brain cells protects against invasion by dangerous toxins and microbes.
Focused ultrasound has shown promise in recent studies for temporarily opening the blood-brain barrier. Here, microscopic inert gas-filled bubbles are injected into the patient’s bloodstream. With MRI guidance, brain regions are targeted while the injected microbubbles circulate.
Woodworth and colleagues previously found that opening the blood-brain barrier temporarily can be performed safely and feasibly in brain tumor patients.
For their current study, the researchers recruited 34 patients to undergo MRI-guided focused ultrasound and then chemotherapy (after opening the blood-brain barrier). The team compared results to those of 185 glioblastoma patients who received the standard of care.
The video shows the process of using microbubbles for MRgFUS procedures to open the blood-brain barrier for chemotherapy delivery. Credit: University of Maryland Medical Center
It treated the trial participants with surgery to remove their brain tumor, followed by six weeks of chemotherapy and radiation, and up to six monthly focused ultrasound treatments plus temozolomide. The group performed 144 treatment cycles on the participants.
The investigators recorded 176 adverse events during the study. These included the following: 87 related to undergoing the focused ultrasound procedure, 54 chemotherapy-related, 25 unrelated, and 10 disease-related. Of the procedure-related adverse events, 40 were grade 1, 46 were grade 2, and one was grade 3.
Among the trial participants, the team reported 13 adverse events with severity grades 3 to 5. These occurred in seven participants. The two grade 5 events were disease-related.
However, no treatment-related deaths occurred during the trial and the group could visualize blood-brain barrier openings in all treatments.
Woodworth and colleagues also reported a median overall survival of 31.3 months and a median progression-free survival of 13.5 months. The control group, meanwhile, had a median progression-free survival of 8.1 months (p = 0.02).
The study authors highlighted that these findings provide “a rationale and strategy for randomized controlled trials” focusing on this procedure for high-grade gliomas with adjuvant chemotherapy. This includes drugs that have previously been considered less brain-penetrant.
“Accordingly, adding a new treatment-augmenting modality to chemotherapy that offers enhanced, localized therapeutic effects in non-enhancing, tumor-infiltrated brain regions after most of the disease has been controlled with surgery and chemoradiotherapy has high potential value,” the authors wrote.
Read the full study here.