Medivis has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Cranial Navigation augmented reality (AR) system.
This is Medivis' second major FDA clearance this year, following the launch of Spine Navigation platform.
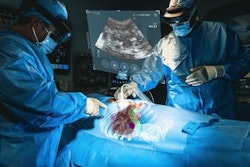
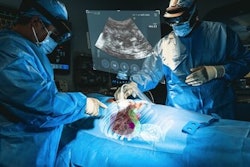
Cranial Navigation is the first AR system to be cleared for intraoperative guidance in cranial neurosurgery, according to Medivis. It uses AR to spatially map patient imaging within the operative field, giving surgeons a real-time view of critical anatomy and planned trajectories.
Cranial Navigation’s portable design allows for bedside use in a wider range of clinical environments. Early clinical data show that the real-time, AR-guided visualization Cranial Navigation provides significantly reduces the rate of external ventricular drain (EVD) misplacement, the firm said.
FDA clearances for Cranial Navigation and Spine Navigation can support reimbursement under established CPT add-on codes 61781 and 61783, respectively, it added.