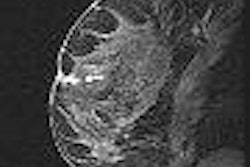
Interventional device developer Ethicon Endo-Surgery has released Mammotome MR, an MRI-guided breast biopsy device, in the U.S.
The product utilizes a biopsy needle equipped with a vacuum device that uses MRI guidance to partially or completely cut, vacuum, and remove suspicious breast tissue for evaluation, according to the Cincinnati-based firm. The procedure can be performed on an outpatient basis, the company said.
By AuntMinnie.com staff writers
May 25, 2006
Related Reading
Neoprobe renews Ethicon deal, March 13, 2006
GE signs biopsy pact with Ethicon, October 25, 2005
Ethicon hits Mammotome milestone, July 21, 2004
Ethicon Endo-Surgery expands Mammotome use, November 26, 2003
Ethicon supports RT-related research, September 18, 2002
Copyright © 2006 AuntMinnie.com