(Booth 2015) Cleveland's MIMvista will offer up a range of advanced visualization applications to visitors at its RSNA booth.
The company has added an epilepsy analysis tool for its MIMneuro package for the quantitative analysis of PET and SPECT brain studies. The software performs ictal-interictal SPECT subtractions and MR coregistration in a few steps, all from one diagnostic reading station, according to the company.
The software's workflow automates the fusion, normalization, subtraction, and display of multiple datasets, while an integrated brain atlas increases confidence in anatomical localization, the company said. The software's automated system highlights significant foci by cluster analysis, providing radiologists and their referring physicians with a clear picture of the epileptic focus. The software began shipping in the second quarter of 2008.
MIMvista has added a serial exam review feature to its MIMcontouring Advanced application. Exams from multiple modalities (CT, MR, PET, and SPECT) can be automatically linked or fused for comparison. Triangulation of fused or linked exams allows corresponding abnormalities to be easily tracked between time points.
Nonrigid differences between serial exams can be overcome using the CT-to-CT deformable registration provided in the software's VoxAlign deformation engine. All contours can be saved as RT Structs and sent to radiation therapy planning systems.
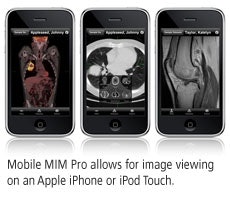
The company also will discuss Mobile MIM Pro, a work-in-progress application that allows for remote image viewing on Apple's iPhone or iPod Touch. It includes zoom, fusion, blending, window/level, standard uptake value, measurement tools, and contour review, according to MIMvista. Mobile MIM Pro is pending U.S. Food and Drug Administration 510(k) clearance and is expected to be available in 2009.