Advanced visualization firm Visage Imaging has received U.S. Food and Drug Administration (FDA) 510(k) approval for its Visage CS 3.1 thin-client software release.
The release brings enhancements to existing cardiac and other capabilities, as well as new optional neuroradiology and oncology applications, according to the Carlsbad, CA-based vendor.
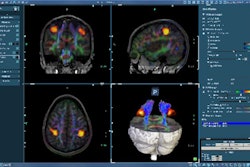
The software's Neuro option provides CT and MR brain perfusion analysis, while an Oncology option includes tools for analyzing, documenting, and comparing lesions for multiple modalities, Visage said.
In addition to the new applications, Visage has incorporated numerous new and streamlined measurement and postprocessing tools such as advanced 3D segmentation, region of interest-based analysis, and time-value curves, according to the vendor.
Visage said it has also improved editing of cardiac left ventricular models.
Related Reading
Visage, Viatronix ink marketing deal, November 25, 2008
Road to RSNA, Advanced Visualization, Visage Imaging, November 6, 2008
Visage wins contract with ACR, October 2, 2008
Visage to show new cardiac tools, June 18, 2008
Visage signs Tufts contract, May 13, 2008
Copyright © 2008 AuntMinnie.com