Dutch advanced visualization software developer MRIguidance has received U.S. Food and Drug Administration (FDA) clearance for its BoneMRI image processing software.
Designed to assist in diagnosis and treatment planning, BoneMRI generates a 3D CT-like image from an MRI scan to enhance visualization of bone structures, according to the company, which is a spin-off from the University of Utrecht. The software can be integrated into the radiological workflow, MRIguidance said.
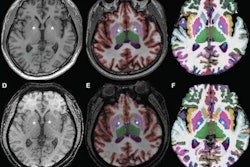
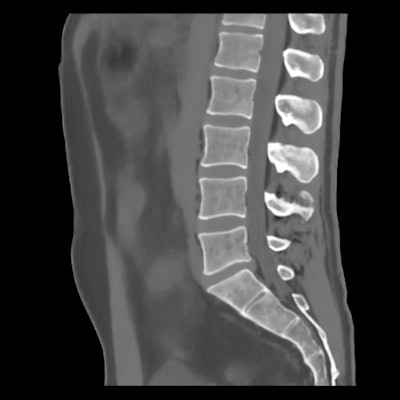
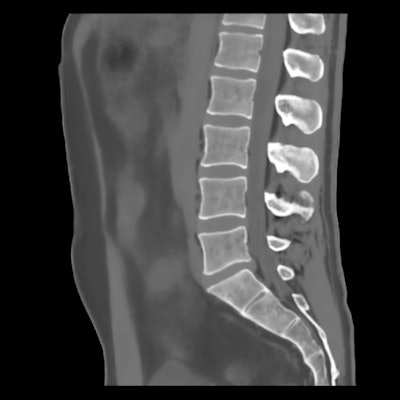 BoneMRI generates 3D MRI images aimed at enhancing visualization of bone structures. Image courtesy of MRIguidance.
BoneMRI generates 3D MRI images aimed at enhancing visualization of bone structures. Image courtesy of MRIguidance.The FDA clearance covers the use of the software in the pelvic region, including the bony anatomy of the sacrum, hip bones, and femoral heads. The company said it also intends to extend the clearance to include the spine later this year.