Matakina International said it has received a medical device license by the Medical Devices Bureau of Health Canada to sell and market its Volpara breast imaging software in the country.
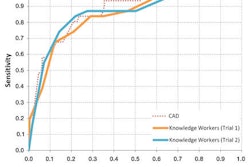
Volpara calculates volumetric breast density as a ratio of fibroglandular tissue and total breast volume estimates. These numerical values help radiologists assess breast tissue composition. Volpara has been cleared by the U.S. Food and Drug Administration (FDA) to provide objective volumetric breast density values, according to the firm.