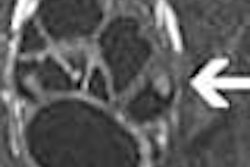
Video-capsule manufacturer Given Imaging is planning to launch its M2A Patency System at the United European Gastroenterology Week conference in Madrid next month. The product is designed to definitively diagnose the presence of obstructing strictures and adhesions in the genitourinary tract, said the Yoqneam, Israel-based firm.
The Patency System consists of an ingestible, dissolvable capsule that is the same size as the M2A capsule endoscope. It includes a radiofrequency ID tag and a handheld scanner used to determine a signal from the tag. Additionally, the capsule contains barium, so that in those instances where the capsule is not excreted after ingestion, a physician may detect the presence of the device with the handheld scanner, and then also determine the exact location of the obstruction under fluoroscopy. If obstructed, the device is designed to dissolve after two to three days and pass naturally, according to the company.
By AuntMinnie.com staff writersOctober 7, 2003
Related Reading
New CPT code for capsule endoscopy, October 2, 2003
Given Imaging gets Australian reimbursement, September 18, 2003
Given Imaging receives FDA first-line tool clearance, July 8, 2003
Given Imaging gets Israeli reimbursement, July 3, 2003
Given Imaging increases revenues, trims loss, May 2, 2003
Copyright © 2003 AuntMinnie.com