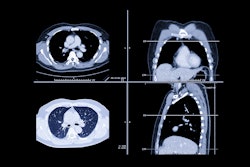
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Brainomix for a patented feature that enables assessment of ischemic core volume on non-contrast CT (NCCT) scans with its AI-enabled 360 Stroke software.
The approval will allow healthcare providers to use Brainomix’s 360 platform to estimate ischemic core volume (the amount of brain tissue affected by a stroke) from routinely available NCCT scans, the Oxford, U.K.-based Brainomix said in a statement, with the goal of improved decision-making about triage and treatment for stroke patients.
Brainomix said that in a study incorporating national-level data from more than 450,000 patients over a three-year period, 360 was associated with a more than 50% increase in mechanical thrombectomy rates.