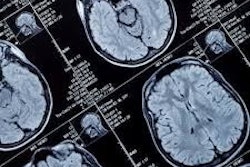
Despite reported issues with CT scanning interfering with certain medical devices, the U.S. Food and Drug Administration (FDA) has said CT is still the preferred imaging modality for patients with medical devices.
In an October 15 communication, the agency said it had received reports of electronic medical devices being damaged during CT scans due to radiation.
"Interference is when the radiation and the device electronics are incompatible, and the resulting damage causes the device to fail to work normally," the FDA said. However, "for imaging procedures specifically, CT continues to be the preferred tomographic imaging technology for people with implantable or wearable medical devices. CT is safer than magnetic resonance imaging (MRI) for people with devices of unknown MRI safety status."
The FDA released the guidance -- which is mostly specific to CT -- noting that it is not aware of any confirmed interference from other imaging technologies such as x-ray, fluoroscopy, angiograms, or mammograms.
Read the FDA's full guidance here.