Immunoglobulin Imaging for Infection/Inflammation
General discussion of Antibodies:
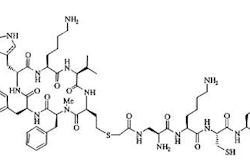
Antibodies are complex proteins composed of 4 polypeptide chains which are designated light and heavy chains and are joined to one another by disulfide bonds. The chains resemble a capital "Y" which has been cleaved vertically into a right and left half, then re-joined by the disulfide bonds. The portion of the molecule at the "bend" in the "Y" is referred to as the hinge region. Each chain is composed of a variable and a constant region. Light chains consist of one variable and one constant region. Each of the heavy chains have 4 to 5 domains (one variable and 3 to 4 constant regions). The variable regions are responsible for antigenic binding. Each of the variable regions of the heavy and light chains contain 3 different hypervariable regions that make up the unique antigen binding site of the molecule. The constant region within a subclass of antibodies contains an amino acid sequence that is highly conserved or similar. This region is referred to as the 'constant' or Fc region and it is responsible for effector functions such as complement fixation and antibody dependent cell cytotoxicity. Antibodies of the IgG class contain carbohydrate in the domain below the hinge region. These sites are commonly used for binding the radiolabel.
Antibodies can be labeled by attachment of a radiopharmaceutical to either the amino acid groups or the carbohydrate moieties of the heavy chains; this process is referred to as conjugation. Isotopes of iodine have generally been incorporated directly into the monoclonal antibody by iodination of the tyrosine residues. The drawback of this method is the random attachment of the agent to various amino-acid residues, including those situated at the antigen binding sites which may alter the properties of the antibody in-vivo. There is also dehalogenation of the label in the liver, spleen, and kidneys which results in excess free radioiodine which poses both dosimetry and image degradation problems. The method of choice for labeling antibodies with radionuclides is by site specific conjugation. In this process a second molecule, such as DTPA for In-111, is chemically bound to the oligosaccharide moiety on the antibody molecule. Because transferrin has a higher binding affinity for indium than DTPA, transchelation can occur in vivo, resulting in a gradual loss of indium from the antibody and redistribution to the liver, spleen, and bone marrow (typical of indium transferrin complexes). Technetium is generally bound by reducing the tracer to a more reactive state and then exchanging it onto native chelating groups, such as free sulfhydryls present on the Fab' antibody fragments. Losses of antibody immunoreactivity occur frequently as a result of the radiolabeling process.
A major determinant of the rate at which a protein leaves the vascular space is its molecular size. Antibody fragments are significantly smaller than whole antibodies and therefore diffuse more rapidly out of the blood stream and into the tissues. Digestion with pepsin removes part of the constant Fc portion to produce an F(ab')2 fragment, which can further be split into two Fab' fragments. Papain digestion splits the molecule into an Fc, and two Fab fragments. Fab fragments are cleared 30 times more rapidly by the kidney compared to whole antibody. Because Fab and Fab' fragments are cleared by the glomerulus but resorbed in the renal tubule, retention of the radiopharmaceutical in the tubules results in relatively high radiation doses to the kidneys. In some instances the whole antibody may be more desirable for imaging because it allows increased time for scanning. IgM antibodies, which are larger than IgG species, remain in the blood pool much longer.
Chimeric antibodies are composed of murine heavy and light chain variable regions attached to a human constant region in order to decrease immunogenicity. Humanized antibodies are made by inserting murine hypervariable regions into the human molecule, again to decrease immunogenicity. Single chain antigen binding proteins consist of the variable domain proteins from both the heavy and light chains. Absence of the Fc region decreases immunogenicity, while maintaining specific antigenic properties. VH- domain proteins are made up of a single heavy chain variable region and unfortunately, have less antigen binding affinity than the parent antibody. Hypervariable region peptides are amino acid sequences which have been synthesized from the hypervariable regions of the heavy chain. Because of their small size, they are cleared rapidly from the circulation and can reach sites no normally accessible to larger Mabs.
Weights of antibodies and antibody fragments (in daltons) are as follows:
- Fab: 50,000
- Fc Fragment: 50,000
- F(ab')2: 100,000
- Whole Ab: 150,000
- Light chain: 23,000
- Heavy chain: 53,000
Complications of Antibody Imaging:
One risk associated with the administration of antibodies (foreign protein) is a severe systemic reaction, but this is rare (less than 1% of patients). Reactions are treated by stopping the infusion and administering an antihistamine or epinephrine if necessary. IgG infusion is contraindicated in patients with selective IgA deficiency who possess antibodies to IgG.
Polyclonal IgG Antibodies:
Chemistry, Pharmacology and Distribution
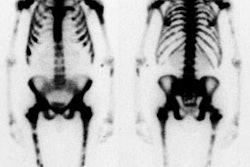
Polyclonal IgG is a non-antigen specific IgG antibody. The radiopharmaceutical is In-111. 1.5 mCi is labeled to one gram of polyclonal IgG. The liver is the critical organ [1.42 rad/mCi] and splenic uptake (about 1% of the administered dose) is much less than that seen with labeled white blood cells. About 15% of the dose is eliminated via the urine [7]. Physiologic uptake is seen in the liver, spleen, bone marrow, and kidney/bladder and this can lead to difficulty in localizing small foci of infection near these organs. Blood pool activity is also seen and may make diagnosis of infected vascular grafts and mycotic aneurysms difficult. Variable GI activity can be seen due to gastrointestinal leakage of protein or bleeding, but generally there is only minimal physiologic bowel activity (1% of the total dose is excreted in the feces). On early images activity may also be seen in the lungs, nose, vagina, and external male genitalia- this activity will decrease on delayed images in the absence of infection. In-111 IgG clears from the blood pool with a half-life of about 12 hours [1].
Advantages of human polyclonal IgG include simple preparation (kit) and a potential for imaging as soon as 6 hours after injection. Also, as granulocytes are not required for labeling, this agent may have advantages in the neutropenic patient. Technetium labeled to IgG has not performed as well particularly at sites of chronic infection. This is most likely due to significant loss of activity due to radioactive decay prior to adequate agent localization at sites of inflammation.
A disadvantage of the agent is its accumulation at sites of sterile inflammatory processes such as hematomas, inflammatory bowel disease, synovitis, and recent fractures (less than 3 weeks old). Indeed this is true of all agents used to detect infection; they are best considered inflammation localizers. Uptake has also been identified in surgical wounds, ostomies/recent fractures (under 3 weeks old), intramuscular injection sites, renal transplant rejection, pancreatitis, myocardial or cerebral infarction, DVT, and pulmonary embolism.
Proposed mechanisms for the accumulation of polyclonal IgG at sites of infection/inflammation include:
- Binding to Fc receptors expressed by cells involved in the inflammatory response (macrophages, PMN's, lymphocytes), but this does not explain why the exam still has a high sensitivity in neutropenic patients.
- Increased vascular permeability at inflammatory sites with an increased extracellular fluid space.
- Direct binding of the IgG or Fc portion to bacteria
Polyclonal IgG Imaging for Infection:
Sensitivity and specificity are similar to WBC imaging (about 90% and 95% respectively). Antibiotics, anti-inflammatory agents, steroids, diabetes, decreased renal function, and chronicity of the infection do not appear to affect the sensitivity of the exam [2,3] Diffuse pulmonary accumulation of the tracer is the most common finding in patients with pneumocystis pneumonia. In the evaluation of HIV patients for infection, no intrapulmonary accumulation of the tracer was seen in patients with Kaposi's sarcoma, or intrathoracic lymphoma [4].
Monoclonal Antigranulocyte Antibodies:
A disadvantage of leukocyte imaging is the labeling process itself- requiring time, handling of blood products, and skilled personnel [9]. Antigranulocyte antibodies whould seem to hold promise for infection imaging. The antibodies are murine derived. After affixing to the cell, the antibody does not influence the normal physiologic capacities of human granulocytes. Only about 10% of the injected radiolabeled antibody is bound to circulating granulocytes, while about 20% circulates as free IgG. Uptake at sites of infection is therefore related to migration of antibody labeled circulating granulocytes and non-specific non-antigen related uptake of free antibody. As with polyclonal IgG, uptake of this agent can also be seen at sites of sterile inflammation. Use of this agent may result in the formation of HAMA with resultant risks for allergic reactions, altered biodistribution after repeat injection, and interference with radioimmunoassays. NeutroSpec (Tc-99m-Fanolesomab) was an IgM antigranulocyte antibody that was withdrawn from the market as a result of life-threatening cardiopulmonary events [9].
Tc-99m anti-granulocyte monoclonal murine antibody Fab' fragments have also been developed. This agent can detect infection/inflammation with an accuracy that is comparable to WBC scanning. The benefits of this agent are that positive images can be obtained as early as 1 to 4 hours post injection (rapid localization), it is simple to use, and it does not elicit a HAMA response.
In a meta-analysis monoclonal antibody imaging had a sensitivity of 81% and a specificity of 77%; however, the sensitivity was better for the peripheral skeleton than for the axial skeleton (87% vs 53%) [10]. For the evaluation of joint infection following arthroplasty, a meta-analysis showed a sensitivity and specificity of 83% and 80%, respectively [8].
REFERENCES:
(1) J Nucl Med 1995; Dec, p.2372-2379
(2) Nuclear Medicine Annual 1993; Datz FL. The current status of radionuclide infection imaging. Ed. Freeman LM. Raven Press, Ltd. New York. 47-76
( 3) J Nucl Med 1994; Datz FL, et al. The efficacy of In-111-polyclonal IgG for the detection of infection and inflammation. 35: 74-83
(4) J Nucl Med 1993; Oct., 1621
(5) Semin Nucl Med 1993; Corstens FHM, et al. Radioimmunoconjugates in the detection of infection and inflammation. 23: 148-164
(6) J Nucl Med 1991; Serafini AN, et al. Clinical evaluation of a scintigraphic method for diagnosing inflammations/infections using human indium-111-labeled nonspecific human IgG. 32: 2227-2232
(7) Ortho Clin N Am 1991; Wegener WA, Alavi A. Diagnostic imaging of musculoskeletal infection. Roentgenography; gallium, indium-labeled white blood cell, gammaglobulin, bone scintigraphy; and MRI. 22: 401-417
(8) Radiology 2007; Pakos EE, et al. Prosthesis infection: diagnosis after total joint arthroplasty with antigranulocyte scintigraphy with 99mTc-labeled monocloncal antibodies- a meta-analysis. 242: 101-108
(9) J Nucl Med 2007; Palestro CJ. In vivo leukocyte labeling: the quest continues. 48: 332-333
(10) Radiology 2007; Pakos EE, et al. Osteomyelitis: antigranulocyte scintigraphy with 99mTc radiolabeled monoclonal antibodies for diagnosis- meta-analysis. 245: 732-741