NEW YORK (Reuters Health), Dec 19 - Pain is reduced and signs of disease modification are present when biochemical markers of cartilage degradation are used to assess knee osteoarthritis (OA). However, the bisphosphonate does not slow disease progression on radiography, investigators with the Knee Osteoarthritis Structural Arthritis (KOSTAR) study reported the November issue of Arthritis and Rheumatism.
The KOSTAR study group consisted of 2,483 patients with medial compartment knee OA and 2-4 mm joint space width on radiography.
Patients were enrolled in one of two 2-year studies, one in North America and one in Europe, evaluating risedronate 5 mg/day, 15 mg/day, 35 mg/week (Europe) and 50 mg/week (North America) compared with placebo. Study endpoints were a reduction in signs and symptoms of OA, a reduction in patient global assessment scores and a slowing of disease progression on radiography.
There was an approximate 20% reduction in signs and symptoms observed and in patient global assessment scores for all patients, those on active treatment and those on placebo.
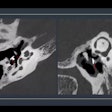
No treatment effect of risedronate was seen in overt signs of osteoarthritis, principal investigator Dr. Clifton O. Bingham, III, of Johns Hopkins University, Baltimore, told Reuters Health. There was no significant slowing of disease progression on radiography, using a definition of a loss of joint space of 0.6 mm or more.
However, risedronate was associated with a dose-dependent reduction in the level of C-terminal cross-linking telopeptide of type II collagen, the investigators report. This is a marker of cartilage degradation seen in progressive OA.
"I certainly would not advocate the use of risedronate for knee osteoarthritis based on this study," Dr. Bingham asserted, "even though it may be having a positive impact on the overall health of the joint."
The problem with controlling disease progression is three-fold, Dr. Bingham said. "The challenge is to identify risk factors for patients with the highest risk of disease progression...We need a more precise evaluation of disease progression, and MRI may be more sensitive...and we need to understand the relation between biochemical markers and radiographic or structural changes," Dr. Bingham said.
"This study and the glucosamine studies show how robust the placebo effect can be and how important it is to include a placebo group," he continued.
"I prescribe risedronate (for arthritis patients) according to labeling. I don't advocate its off-label use," Dr. Bingham emphasized.
By Martha Kerr
Last Updated: 2006-12-18 15:52:17 -0400 (Reuters Health)
Arthritis and Rheum 2006;54:3494-3507.
Related Reading
Risedronate provides better earlier fracture protection than alendronate, November 23, 2006
Copyright © 2006 Reuters Limited. All rights reserved. Republication or redistribution of Reuters content, including by framing or similar means, is expressly prohibited without the prior written consent of Reuters. Reuters shall not be liable for any errors or delays in the content, or for any actions taken in reliance thereon. Reuters and the Reuters sphere logo are registered trademarks and trademarks of the Reuters group of companies around the world.