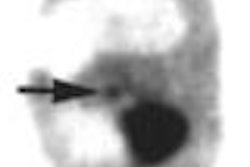
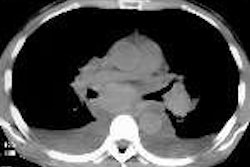
Palatin Technologies and Walter Reed Army Medical Center will work together to test the Princeton, NJ-based vendor's LeuTech investigational infection imaging agent for the early detection of inhalation anthrax.
Under the agreement, study patients exposed to anthrax will begin receiving LeuTech scans this week. LeuTech is a radiolabeled monoclonal antibody that binds to white blood cells that collect at infection sites, allowing for detection with a gamma camera, according to Palatin.
The agreement was made through the Henry M. Jackson Foundation for the Advancement of Military Medicine. If initial results of this clinical trial demonstrate LeuTech's utility in the diagnosis of inhalation anthrax, Palatin said it would initiate discussions with the Food and Drug Administration to provide LeuTech to other medical institutions under the regulatory provision of a treatment investigational new drug application (IND).
By AuntMinnie.com staff writersNovember 5, 2001
Related Reading
FDA wants more data on Palatin’s LeuTech agent, September 28, 2000
Copyright © 2001 AuntMinnie.com