Contrast agent manufacturer Guerbet has received approval from the U.S. Food and Drug Administration (FDA) for a new indication for its Lipiodol contrast agent.
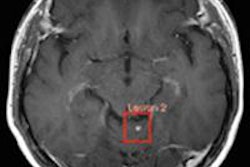
Lipiodol has been approved for selective hepatic intra-arterial use for imaging tumors in adults with known hepatocellular carcinoma (HCC), the most common primary liver tumor and the third-leading cause of cancer-related death worldwide, according to Guerbet.
The announcement comes soon after the FDA granted approval to manufacture Lipiodol for U.S. distribution at a Jubilant HollisterStier site in Canada, the company said.
Lipiodol is now indicated for hysterosalpingography in adults, lymphography in adults and children, and selective hepatic intra-arterial use for imaging tumors in adults with known HCC.