DUBAI - The development of effective imaging biomarkers can spark a huge improvement in the diagnosis, management, and follow-up of patients with multiple sclerosis (MS), but much greater standardization is required, ECR 2017 President Dr. Paul Parizel, PhD, told delegates at the Arab Health congress on Tuesday.
More standardized MRI acquisition protocols, along with better quantitative reporting tools, provides a much better understanding of the natural history of MS and allows accurate treatment monitoring for the greater benefit of patients, he explained.
 Standardization is the future, Parizel told Arab Health 2017 attendees.
Standardization is the future, Parizel told Arab Health 2017 attendees."Standardization is key," noted Parizel, who is president of the European Society of Radiology (ESR) and professor and chair in the radiology department at Antwerp University Hospital (UZA) in Belgium. "Standardization is where I believe radiology is going to move in the next decade."
Speaking on day two of the 16th Total Radiology conference at Arab Health 2017, Parizel explained that in data acquisition for MRI, there are significant differences in image acquisition; a study done on one vendor's machine may be different from that done on another company's system. In addition, there are many different ways to process or interpret an image.
"You could give the same dataset to 10 different radiologists and get 10 different opinions. And every single radiologist is convinced that he or she is the best radiologist in the world," he explained, pointing out these differences can be avoided by homogenizing acquisition parameters, even though equipment from different vendors is used, and by standardizing all information.
Today it's feasible to combine the objective measurements of computers with the advanced expertise and experience of radiologists. This should not be perceived as a challenge or a threat to the radiological community at large, but rather as an opportunity to improve our practice, he commented.
Parizel explained when we're not feeling well and we go to the doctor, the doctor may take a peripheral blood sample and the sample is always treated in the same way -- it goes into the test tube, it's placed into the centrifuge, specific measurements are made, and we get a report with numbers that indicate what the normal range is and our results are then compared against this range.
"I believe that we are going in the same direction with MR imaging and that we should consider MR as a lab," he said. "This would allow us to feed the images into a computer and produce a data report showing the exact volume of brain and volume of lesions, with normal range and normal values matched for age and sex."
The switch from black and white
Parizel compared the attitude toward the paradigm shift in MR acquisition protocols to the advent of color TV in the 1960s when many people queried the need for color TV when black and white worked fine. Now everyone is watching color TV and nobody would want to go back to black and white.
"We are moving from an era where we are simply interpreting 2D images in black and white, to volumetric acquisitions in 3D," he said. "We are moving from black and white to color TV."
In the radiology department at UZA, standardization is rapidly becoming the most important measure for radiologists, Parizel continued. For patients who have repetitive scans -- e.g., brain tumor patients or MS patients -- radiologists try to standardize the imaging protocols into obtaining only isotropic volumetric 3D acquisitions.
"At the request of the neurologist, we can feed the data from MS patients into volumetric analysis software to measure the change between scans," he explained. "This is because a new class of disease-modifying medications for MS patients, known as biologics, represents a huge cost burden to society."
Not all patients benefit from biologics, and these medications have a number of potential side effects and complications, so it is essential to know, from an early stage, whether there is a positive effect. "If it turns out that there are no changes, we can stop the medication and save unnecessary side effects for the patients and cost to society," he said.
Adoption of biomarkers
Moving on to neuroimaging biomarkers, Parizel noted that two types can be distinguished: cross-sectional and longitudinal. In the cross-sectional approach, we extract and measure volumes in a 3D MRI dataset of a single subject, he explained. The volume of the whole brain, or part of the brain (gray matter, white matter, cerebrospinal fluid, hippocampus, etc.), can be computed through segmentation techniques. These methods rely on segmenting brain tissue from the surrounding scalp and other extracerebral tissues, he continued.
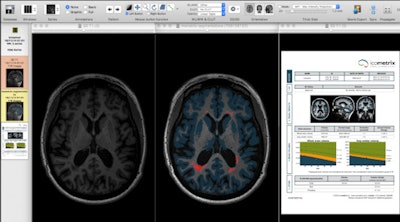 Segmentation techniques make it possible to extract very detailed, quantitative information from 3D datasets of the brain in MS patients. Left: Axial slice of MP-RAGE T1-weighted 3D set through the brain of MS patient. Middle: Image displays same slice after application of segmentation technique, showing MS lesions (or plaques) in red and cortical gray matter in blue. Right: Automatically generated report reveals exact volume of white matter lesions and cortical gray matter, and comparing the values of the patient against age- and sex-matched normal individuals. Such quantitative methods provide accurate follow-up and treatment monitoring of patients with multiple sclerosis because they allow sequential assessment of both white matter lesions and cortical gray matter atrophy. Figure courtesy of Dr. Paul Parizel, PhD.
Segmentation techniques make it possible to extract very detailed, quantitative information from 3D datasets of the brain in MS patients. Left: Axial slice of MP-RAGE T1-weighted 3D set through the brain of MS patient. Middle: Image displays same slice after application of segmentation technique, showing MS lesions (or plaques) in red and cortical gray matter in blue. Right: Automatically generated report reveals exact volume of white matter lesions and cortical gray matter, and comparing the values of the patient against age- and sex-matched normal individuals. Such quantitative methods provide accurate follow-up and treatment monitoring of patients with multiple sclerosis because they allow sequential assessment of both white matter lesions and cortical gray matter atrophy. Figure courtesy of Dr. Paul Parizel, PhD.Longitudinal neuroimaging biomarkers take into account two (or more) MRI scans of the same subject, obtained at different time points, to calculate volume changes in brain volume. This enables assessment of MS patients for progressive brain shrinkage (atrophy), a parameter reflecting neuro-axonal and myelin loss, and it is increasingly being used as an outcome measure in MS treatment trials.
Moreover, longitudinal methods for brain atrophy typically match two MRI scans using registration techniques and directly extract small changes in brain volume from this process. A similar approach can be used for the longitudinal segmentation of white matter lesions, according to Parizel.
A prerequisite for adopting these biomarkers is standardization of image acquisition, data processing, and analysis and image interpretation (i.e., generating a report). MRI protocols and sequences must be reproducible, accurate, and sensitive; quality assessment should be an integral part of this process, he said. Methods used for analysis should be adequate and observer-independent. Ideally it should be possible to compare a biomarker in a single patient with a healthy control group (reference values).
The key words in this chain of production are "standardization" and "validation," and radiologists need to rely on computers to handle large datasets and perform centralized analysis and automated quantification, Parizel explained.
Adding quantitative MRI biomarkers takes clinical neuroimaging to the next level, he pointed out. However, to introduce quantitative biomarkers in the clinical imaging pipeline, several elements must be taken into account, including accuracy and reproducibility, integration into the standard workflow, and scan time (current average MRI protocol for MS patients takes 20-35 minutes).
Biomarkers should help doctors and not generate extra work, Parizel stressed. Radiologists and neurologists don't have time to perform additional postprocessing for every patient, and ideally the postprocessing should be linked to patient informatics, to automatically generate reports that include biomarker information about the lesions and cerebral atrophy.
The radiologist should report back to the neurologist, qualitatively if not quantitatively, about the lesion status and the atrophy of MS patients covering the following: comparison with previous scan(s); evidence of new disease activity; number of new lesions (T2/T1); lesion size; overall assessment, including presence (definite/probable) and extent (number of new/enlarging lesions or gadolinium-enhancing lesions) of disease activity; change in T2 lesion volume; and evidence of brain atrophy, he concluded.