Opening up narrowed veins from the brain and spinal cord -- a procedure called venoplasty -- is not effective for treating multiple sclerosis (MS), according to a study presented on March 8 at the Society of Interventional Radiology (SIR) meeting in Washington, DC, by researchers from the University of British Columbia.
The finding discredits the claim that multiple sclerosis patients can realize significant improvements from a one-time medical procedure, the university said in a statement.
Thousands of people with MS have undergone venoplasty, also referred to as "liberation therapy," since 2009, when it was introduced by Dr. Paolo Zamboni of Italy. Zamboni claimed that the narrowing of veins in the neck could cause iron to accumulate in the brain and spinal cord, triggering an autoimmune response; multiple sclerosis is an autoimmune disease in which the body's own defenses attack the protective coating of brain cells.
However, several research studies since then have questioned whether liberation therapy really works for MS. While some studies have supported Zamboni's findings, others have suggested there could be a placebo effect at work. For example, a 2013 study in the Lancet by Dr. Anthony Traboulsee of the University of British Columbia used angiography and ultrasound in patients with MS but was unable to replicate Zamboni's findings.
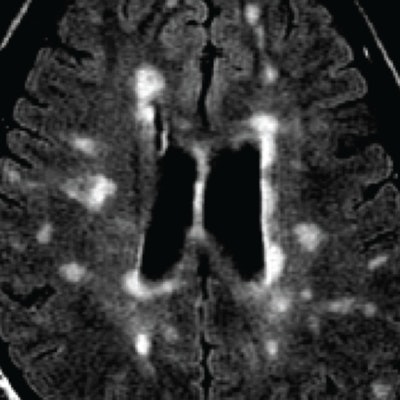
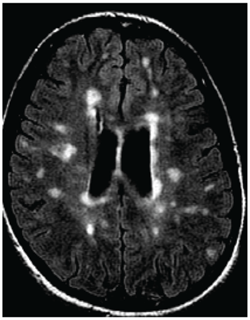 MRI of a person with multiple sclerosis. White areas show permanent scarring caused by the disease. Image courtesy of the University of British Columbia.
MRI of a person with multiple sclerosis. White areas show permanent scarring caused by the disease. Image courtesy of the University of British Columbia.The study presented at SIR 2017 was also led by Traboulsee and included 104 patients. All patients had a catheter inserted into their blocked veins, but only 49 underwent venoplasty, in which their vessel walls were expanded by a small inflated balloon. The study was double-blinded: Neither patients nor the physicians treating them knew who received the actual procedure. The researchers used MRI to evaluate the results.
Traboulsee and colleagues found no statistically significant difference between the treatment group and the placebo group in patient symptoms, either as reported by the patients or as determined by physicians, both three days after the procedure and a year later, the university said.
"We hope these findings, coming from a carefully controlled, 'gold standard' study, will persuade people with multiple sclerosis not to pursue liberation therapy, which is an invasive procedure that carries the risk of complications, as well as significant financial cost," Traboulsee said in the university's statement.
The $5.4 million study was funded by the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, and the provinces of British Columbia, Manitoba, and Quebec. Traboulsee and colleagues are now preparing an article on their findings to be published in a peer-reviewed journal.