An MRI-based predictive model could help guide chemotherapy and immunotherapy for triple-negative breast cancer patients, suggest findings published July 30 in the American Journal of Roentgenology.
The model achieved high marks in predicting complete pathologic response and showed strong agreement between model-predicted outcomes and observed outcomes in triple-negative breast cancer patients, wrote a team led by Changhong Liang, MD, and colleagues from Southern Medical University in Guangzhou, China.
“This … model could facilitate timely tailoring of clinical regimens after immunotherapy initiation by informing optimal deescalation strategies for responders while prompting therapeutic adaptations for nonresponders,” Liang and colleagues wrote.
Triple-negative breast cancer is an aggressive subtype that has a poorer prognosis. The researchers noted a lack of targeted therapies for this breast cancer subtype. While neoadjuvant chemoimmunotherapy improves pathologic complete response rates, it can be challenging to select patients who are good candidates for this treatment.
The Liang team developed and tested an MRI-based model that uses baseline and early-treatment imaging features to predict complete pathologic response in patients undergoing chemotherapy. This also includes dynamic contrast enhancement (DCE) imaging features.
The training set included 90 women, while the external test set included 29 women. Two radiologists evaluated MRI features, including percentage enhancement reduction, which assessed relative expansion of intralesional non-enhancing components after early neoadjuvant chemotherapy.
The team built its model by using independent predictors from a multivariable logistic regression analysis of imaging features. It also used Shapley additive explanations (SHAP) analysis to find how these features contribute to model predictions in the training set.
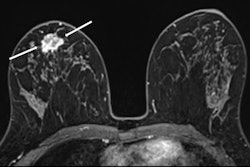
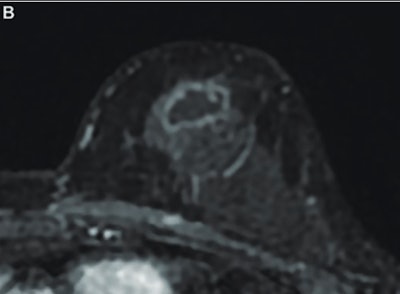
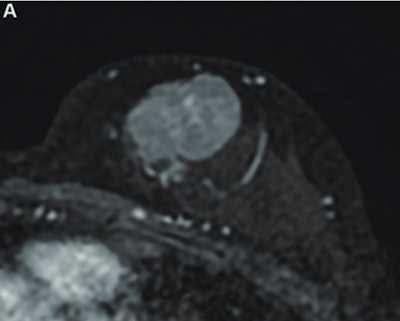 MR image from a 55-year-old patient with triple-negative breast cancer from training set. (A, above) Axial postcontrast T1-weighted image from baseline MRI exam shows unifocal enhancing mass (unifocality: positive). (B, below) Axial postcontrast T1-weighted image from MRI examination per-formed after one cycle of neoadjuvant chemoimmunotherapy shows >37% reduction in lesion size (early tumor shrinkage: positive) and development of non-enhancing region within lesion (percentage enhancement reduction: positive).ARRS
MR image from a 55-year-old patient with triple-negative breast cancer from training set. (A, above) Axial postcontrast T1-weighted image from baseline MRI exam shows unifocal enhancing mass (unifocality: positive). (B, below) Axial postcontrast T1-weighted image from MRI examination per-formed after one cycle of neoadjuvant chemoimmunotherapy shows >37% reduction in lesion size (early tumor shrinkage: positive) and development of non-enhancing region within lesion (percentage enhancement reduction: positive).ARRS
The researchers reported the following independent predictors of complete pathologic response in the training set: tumor unifocality (odds ratio [OR] = 7.2, p = 0.001) on pretreatment MRI, early tumor shrinkage ≥ 37% (OR = 9.7, p < 0.001), and percentage enhancement reduction (OR = 9.7, p < 0.001) on early-chemotherapy MRI.
In the external test set, the resulting model achieved an area under the curve (AUC) of 0.88, 74% sensitivity, and 90% specificity for predicting complete pathologic response. The Hosmer-Lemeshow test showed satisfactory model fit (p = 0.67). The team also reported substantial interobserver agreement for focality (Cohen's kappa coefficient [κ] = 0.7), early tumor shrinkage (κ = 0.66), and percentage enhancement reduction (κ = 0.61).
Finally, in SHAP analysis, global importance for model predictions was highest for percentage enhancement reduction (mean absolute SHAP value, 0.42), followed by early tumor shrinkage (0.32) and unifocality (0.21).
The results underscore how noninvasive MRI markers could have a role in monitoring early immunotherapy response, the authors highlighted.
“The present study’s focus on conventional MRI features offers three key advantages: interpretable parameters that enable biologically plausible understanding; compatibility with standard clinical workflows through routine MRI protocols; and potential synergistic predictive value between baseline tumor characteristics and treatment-emergent dynamic changes observed early after [treatment] initiation,” they added.
The full study can be found here.