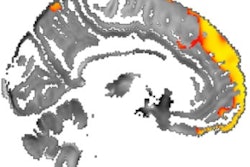
Lower levels of iron in the thalamus as seen on MRI scans of people with multiple sclerosis (MS) are a strong indication that the disease will progress and lead to greater disability, according to a study published online July 17 in Radiology.
Using an MRI technique known as quantitative susceptibility mapping (QSM), researchers from the University at Buffalo (UB) also discovered higher iron content in other deep gray-matter structures in this patient population, also indicating a poor prognosis. The disparity in iron levels is particularly problematic for cellular brain and central nervous system functions, as well as myelination of neurons.
"Our study contributes to the understanding of the dynamics of iron within subcortical gray matter by using sophisticated techniques such as QSM and voxelwise analysis in a large sample of participants with relapsing-remitting MS and secondary progressive MS," the authors wrote. "Our results shed further light on the potential mechanisms underlying thalamic iron reduction as well as iron accumulation within basal ganglia, suggesting that these processes are not merely indirect consequences of structural atrophy."
MS catalysts
For many years, brain atrophy has been considered the primary catalyst in predicting cognitive and physical decline in MS patients, but there are limitations to depending on this characteristic to assess the disease. Brain atrophy takes a long time to detect, and because the disease has already progressed by that time, treatment may be too late to be effective.
More recent studies have described the influence of iron in certain brain regions, as well as white- and gray-matter damage, on the development and sustainability of MS. The thalamus, for example, helps process sensory input by acting as a relay between certain brain structures and the spinal cord.
In that regard, quantitative susceptibility mapping has become a more widely used MR imaging tool to quantify iron in vivo throughout the brain and in specific regions of interest, such as the basal ganglia and the thalamus.
"QSM has been shown to be exquisitely sensitive to paramagnetic and diamagnetic tissue components and can depict specific subcortical gray-matter structures with an excellent level of anatomic detail," wrote lead author Dr. Robert Zivadinov, PhD, a professor of neurology at UB's Jacobs School of Medicine and Biomedical Sciences, and colleagues. "It has yet to be elucidated, however, whether local changes in brain tissue iron concentrations are a causal factor in neurodegeneration or an epiphenomenon of cell death."
QSM protocol
For this prospective study, Zivadinov and colleagues enrolled 600 MS patients (mean age, 45 ± 9.8 years) and 250 healthy controls (mean age, 44.9 ± 14.5 years) between March 2009 and November 2013.
The MS subjects included 452 (75%) with relapsing-remitting disease and 148 (25%) with secondary progressive MS, all of whom were undergoing disease-modifying treatment. The mean disease duration for MS patients was 13.3 years; 11.3 years for relapsing-remitting disease and 19.5 years for secondary progressive MS.
All images were acquired on the same 3-tesla scanner (Signa Excite HD, GE Healthcare) with an eight-channel head and neck coil. The MRI protocol included T2-weighted fast fluid-attenuated inversion-recovery (FLAIR) and 3D single-echo spoiled gradient-echo sequences to measure magnetic susceptibility.
The MR scans were performed within 30 days of a subject's physical and/or neurologic exam. Deep gray-matter susceptibility was measured in parts per billion (ppb) and analyzed via region-of-interest and voxelwise methods.
The degree of each subject's disability was evaluated on the Expanded Disability Status Scale (EDSS). With the scale, the lower the rating, the less the disability: For example, MS patients between 1.0 and 4.5 are able to walk without assistance. Patients with a score above 5 have increasing difficulty in walking.
QSM evaluations
As one might expect, MS patients had significantly greater T2 lesion volume, as well as lower volumes of white matter, gray matter, and deep gray matter, compared with the healthy controls. The same results were observed in comparisons of MS patients with relapsing-remitting disease and secondary progressive MS.
| Volume comparisons between MS patients and healthy control subjects | ||||||
| Volume | Healthy controls (mL) | MS patients (mL) | p-value* | Patients with relapsing-remitting MS (mL) | Patients with secondary progressive MS (mL) | p-value* |
| Lesion | 0.7 ± 4.2 | 15.0 ± 18.9 | < 0.001 | 13.0 ± 17.2 | 21.1 ± 22.5 | < 0.001 |
| Gray matter | 769.6 ± 55.6 | 744.8 ± 58.7 | < 0.001 | 755.8 ± 54.9 | 711.4 ± 57.1 | < 0.001 |
| White matter | 755.6 ± 41.9 | 721.8 ± 51.2 | < 0.001 | 728.2 ± 50.1 | 702.2 ± 49.3 | < 0.001 |
Relative to QSM, the researchers found that MS patients had significantly lower thalamic susceptibility and higher susceptibility in the basal ganglia, caudate, putamen, and globus pallidus, compared with the healthy controls. There also were significant differences in several QSM comparisons between relapsing-remitting MS and secondary progressive MS patients and healthy controls.
| QSM values for MS patients and healthy control subjects | ||||||
| Brain region | Healthy controls (ppb) | MS patients (ppb) | p-value | Patients with relapsing-remitting MS (ppb) | Patients with secondary progressive MS (ppb) | p-value |
| Basal ganglia | 54.8 ± 11.9 | 62.0 ± 13.2 | < 0.001* | 60.5 ± 12.4 | 66.7 ± 14.6 | 0.041* |
| Thalamus | -1.1 6.3 | -7.5 ± 8.7 | < 0.001* | -6.4 ± 8.1 | -10.09 ± 9.3 | 0.011* |
| Caudate | 37.2 ± 10.3 | 44.5 ± 12.1 | < 0.001* | 43.6 ± 11.9 | 47.4 ± 12.2 | 0.35 |
| Putamen | 47.9 ± 15.2 | 51.9 ± 14.9 | < 0.001* | 50.6 ± 14.1 | 55.8 ± 16.88 | 0.12 |
| Globus pallidus | 107.3 ± 20.1 | 124.3 ± 25.7 | < 0.001* | 121.8 ± 24.9 | 132.1 ± 26.6 | 0.08 |
ppb = parts per billion.
As for the clinical implications, lower iron content in the thalamus and higher iron content in other deep gray-matter structures were associated with significant adverse effects in MS patients, who experienced longer disease duration (13.3 ± 9.2 years) and a higher degree of disability (2.5 on the EDSS; range: 1.5-5.0).
"These findings seem to suggest that although thalamic atrophy is known to occur since the early stages of disease, and as such may partially contribute to iron changes, some other mechanisms are involved," Zivadinov and colleagues wrote. "We showed that even after correcting for structural tissue volume in the voxelwise analysis, susceptibility was still lower in the MS cohort as a whole and in participants with secondary progressive MS compared with those with relapsing-remitting MS."