An MRI technique known as quantitative susceptibility mapping (QSM) reveals that patients with Parkinson's disease have increased iron deposition in the areas of the brain that handle cognition, memory, and motor function, according to a study published February 20 in the Journal of Neurology, Neurosurgery, and Psychiatry.
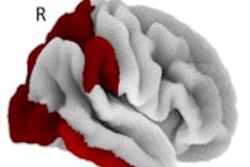
QSM led to the discovery of elevated concentrations of iron in the hippocampus, thalamus, and parietal and prefrontal cortices that were all associated with lower cognition and memory, according to the researchers, led by Dr. Rimona Weil of University College London (UCL). Increased iron deposits were similarly observed in the putamen of study participants that had weaker motor function.
"These anatomically specific changes were detected where conventional neuroimaging failed to identify atrophy and without requiring predefined regions of interest," the authors wrote. "Our findings have important potential as neuroimaging markers of disease activity with application in the clinic and in therapeutic trials."
Recent research suggests that increased iron deposition in key brain regions could be a contributor to cognitive dysfunction. Interestingly, iron accumulates in the brain as part of the natural aging process, especially in the basal ganglia, which is associated with motor skills. To get a better handle on a possible connection with cognitive dysfunction, researchers have begun to use QSM to measure iron content in the brain against people's cognition and movement.
Although QSM previously has been used to measure iron deposits in the basal ganglia, the sequence has never been used across the whole brain to track cognitive changes in patients with Parkinson's disease, according to the researchers. Hence, they turned to the technique to test their hypothesis that iron deposits would be higher in brain regions associated with poorer cognitive ability, particularly in the areas where Parkinson's disease dementia is commonly prevalent.
The researchers recruited 100 patients (mean age, 66.4 ± 7.7 years; range, 49-80 years) who had been diagnosed with Parkinson's disease within the previous 10 years at UCL's facility between October 2017 and December 2018. In addition, 37 age-matched healthy controls (mean age, 66.1 ± 9.4 years; range, 50-80 years) were enrolled into the cohort. Participants underwent 3-tesla MRI scans (Prisma, Siemens Healthineers) with a protocol that included QSM, susceptibility-weighted, and T1-weighted sequences.
Compared with healthy controls, the MR images showed significant QSM increases (p < 0.05) consistent with higher iron deposits in the prefrontal cortex and putamen of patients with Parkinson's disease. In addition, whole-brain analyses found that QSM increases in the hippocampus and thalamus correlated with lower cognitive performance and reduced visual function, while greater levels of iron in the putamen related to poorer motor function.
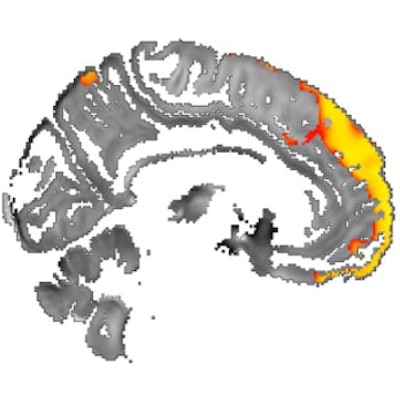
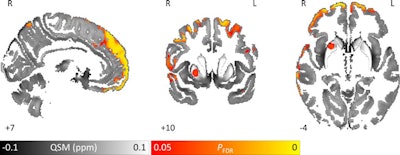 Images show area of significantly higher iron concentration in the brains of Parkinson's disease patients, compared with healthy controls. Whole-brain results are overlaid onto the QSM template in the Montreal Neurological Institute (MNI) coordinate system. Red/yellow clusters represent statistically significant differences, as indicated by the color bar. Numbers represent position of slice in millimeters in MNI coordinate space. FDR: False discovery rate. Images courtesy of Journal of Neurology, Neurosurgery, and Psychiatry.
Images show area of significantly higher iron concentration in the brains of Parkinson's disease patients, compared with healthy controls. Whole-brain results are overlaid onto the QSM template in the Montreal Neurological Institute (MNI) coordinate system. Red/yellow clusters represent statistically significant differences, as indicated by the color bar. Numbers represent position of slice in millimeters in MNI coordinate space. FDR: False discovery rate. Images courtesy of Journal of Neurology, Neurosurgery, and Psychiatry.QSM's ability to correlate iron deposits in these brain regions is clinically noteworthy because it suggests that the MRI sequence is "more sensitive to early tissue changes than conventional atrophy measurements," the authors noted. As a result, QSM could help determine earlier which patients might be at risk for future cognitive decline and identify "neuroimaging markers of disease activity with application in the clinic and in therapeutic trials."