Virtual colonoscopy studies using intravenous glucagon or Buscopan continue to generate mixed results concerning their ability to optimize colonic distension, a key factor in acquiring diagnostic CT data. In fact, two recent European trials came to opposing conclusions on the issue of distension.
First, a German group concluded that Buscopan, but not glucagon, improved colonic distension significantly, adding their findings to a mixed bag of previous results on the subject. Two earlier studies showed no distension benefit from glucagon.
However, radiologists from Rome -- who saw no significant distension improvement using either agent -- found that patients who received either drug reported far less discomfort, and were far more willing to undergo a future VC exam, than patients who received only a placebo. Willingness to undergo periodic examination is, of course, key to the success of any screening program. It is particularly relevant to colorectal cancer screening, which suffers from low compliance.
In any case, the drugs aren't a free ride. In differing degrees, the intravenously administered agents add cost, risk, and staff time to virtual colonoscopy screening -- an exam that many radiologists believe should remain as simple and noninvasive as possible, lest it lose some key advantages over conventional colonoscopy. Of the two agents, butylscopolaminebromide (Buscopan, Boehringer Ingelheim, Ingelheim, Germany) is cheaper but contraindicated in more patients. It is approved for human use in Europe and Australia, but not in the U.S.
Berlin study measures distension
"Contraindications to butylscopalamine (Buscopan) include prostate hyperplasia, clinically relevant tachycardia, and increased intraocular pressure," explained Dr. Patrick Hein from Berlin's Charité Hospital and Humboldt University at the 2004 European Congress of Radiology. "The effect of butylscopalamine is reduction of the adaptability of the eyes, which especially compromises driving immediately after the application or examination. Contraindications to glucagon (hydrochloride) include acute hypersensitivity reactions and the presence of acute pheochromocytoma." Glucagon is also the more expensive drug, at about $40 a dose, he said.
Still, most patients suffer no serious ill effects from either agent, leaving the question of whether they are indeed worth the cost and staff time. Hein, along with principal investigator Dr. Noga Rogalla, Dr. Alexander Lembcke, Dr. Eike Hein, and colleagues examined 150 patients undergoing virtual colonoscopy to determine whether either antispasmodic agent improved colonic distension.
"Diagnostic quality relies on complete bowel distension," Hein said. "Physical distension of the bowel lumen with CO2 or air is often limited by patients' discomfort. Pharmacologic distension of the bowel wall has been proven to be effective in the diagnostic assessment of the bowel."
The researchers examined 150 patients with similar anthropomorphic data, abdominal volume, and diagnostic indications, who were divided into three groups: group 1 (n=50) received a (saline) placebo intravenously, group 2 (n=50) received 1 mg glucagon (GlucaGen, Novo Nordisk, Bagsvaerd, Denmark), and group 3 (n=50) received 20 mg of IV Buscopan. Any patients with contraindications to the agents were sent to group 1.
The subjects underwent cathartic bowel cleansing with a phospho-soda prep the day before the exam. Prior to CT they also received an oral contrast agent. Manual colon insufflation with CO2 preceded administration of the IV contrast agent iopromide (Ultravist 370, Schering, Berlin, Germany), then additional CO2 was introduced to patient tolerance. Supine imaging was performed on an Aquilion 16 MDCT scanner (Toshiba Medical Systems, Tokyo, Japan) set at 16 x 1-mm collimation, pitch 33-60, 90 mAs, and 0.5-sec. rotation time. (The institution scans in prone position only in cases in which supine imaging proves inadequate, Hein told AuntMinnie.com.)
Image volume was calculated from opaque surface views on 3D endoluminal reconstructions on an EasyVision workstation (Philips Medical Systems, Andover, MA). The total length, volume, and radial distensibility were calculated for each group and in each of seven colonic segments, Hein said.
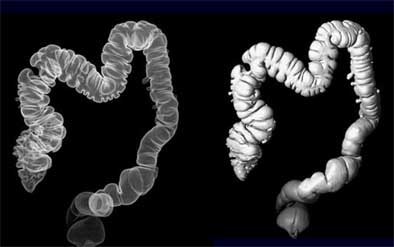 |
| Reconstructed 3D view of the colon (left) and opaque surface view (right). Images (above and top of article) courtesy of Dr. Noga Rogalla and Dr. Patrick Hein. |
"We evaluated parameters (from) the starting point in the cecum and the endpoint in the rectum," he said. "The path through the colon was automatically computed, and the colon length in centimeters was already calculated." In case of stenosis or collapse of a segment, the path was traced manually.
Volume in milliliters (ml) was determined automatically from the 3D reconstruction. "In case of stenosis, the tumor radial distensibility was calculated according to volume," Hein said.
The results showed a small improvement in distension between the glucagon group and the placebo group, while all patients receiving Buscopan had higher values for length, volume, and distension compared to the placebo group. The difference was not significant between the (group 1) placebo and (group 2) glucagon groups (p=0.16), but reached significance between the placebo and (group 3) Buscopan groups (p=0.032), Hein said.
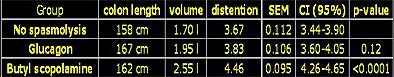 |
| Chart shows results of placebo, glucagon, and Buscopan VC cohorts in terms of colon length, volume in milliliters, and distension. Distension differences were not significant between the placebo and glucagon groups (p=0.16), but did reach significance between the placebo and Buscopan (butylscopalamine) groups (p=0.032). Chart courtesy of Dr. Noga Rogalla and Dr. Patrick Hein. |
"Only two patients in the (Buscopan) group (3) showed collapse of the distal sigmoid colon, necessitating scanning in the prone position," he said. "In group 2 (glucagon), five segments in four patients were collapsed, with one patient showing nondistension of the distal sigmoid colon and part of the ascending colon. In group 1 (placebo), nine patients had to be scanned in the prone position. All nine showed collapse of the distal sigmoid colon, in one case with additional collapse of the proximal sigmoid and two with additional collapse of parts of the descending colon."
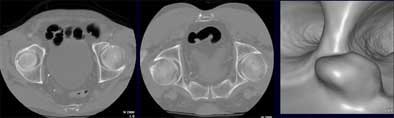 |
| Axial image (left) shows partial collapse in sigmoid colon in supine acquisition. Scanning the patient in prone position (middle) revealed a polyp, shown in reconstructed 3D view (right). Images courtesy of Dr. Noga Rogalla and Dr. Patrick Hein. |
Buscopan can be recommended for use in VC because it "improves colonic distension," Hein concluded. "If contraindications are present, one may resort to glucagon. However, it is less effective and did not produce significantly better results than found in nonpremedicated patients," he said. "In the case of optimal bowel preparation and fecal tagging, the absence of collapsed bowel segments due to spasmolysis reduces the necessity of repeated (CT exams), and thus additional radiation exposure."
Italian group finds patient, not distension, benefits
In the next ECR presentation, Dr. Riccardo Iannaccone from the University of Rome "La Sapienza" noted that VC providers have been administering antiperistaltic drugs, such as Buscopan (in Europe and Australia) and glucagon (in the U.S.), for years to improve colonic distension and patient compliance. But the drugs have generally failed to demonstrate an overall improvement in colonic distension, he said.
"There are two studies in the literature, one from (Dr. Judy) Yee at UCSF and one from (Dr. Martina) Morrin at Beth Israel, that emphasize that glucagon did not improve colonic distension," he said (AJR, July 1999, Vol. 173:1, pp. 169-172; European Radiology, March 2002, Vol. 12:3, pp. 525-530). Last year, Taylor et al did find improved distension using Buscopan (Radiology, October 2003, Vol. 229:1, pp. 99-108), he said, but Bruzzi et al disagreed, concluding that Buscopan was largely ineffective in improving distension, except in select patients such as those with severe diverticulitis (European Radiology, October 2003, Vol. 13:10, pp. 2264-2270).
Still, Iannaccone said, an issue that had not been evaluated (and the subject of the group's present study) was the agents' effect on comfort and patient-compliance aspects of virtual colonoscopy. Toward that end, Iannaccone, along with Dr. Andreas Laghi, Dr. Roberto Passariello, Dr. Carlo Catalano and colleagues, recruited 162 patients who had been referred for virtual colonoscopy, assigning them randomly to one of three groups:
- Group 1, placebo (5 ml IV saline, 0.9% sodium chloride)
- Group 2, glucagon (54 patients, 1 mg IV GlucaGen)
- Group 3, Buscopan (57 patients 20 mg IV hyoscine butylbromide)
The patients underwent a standard cathartic cleansing regimen using either polyethylene glycol or phospho-soda. Manual room-air insufflation was performed to patient tolerance, and all patients were scanned supine and prone on a Somatom Plus 4 Volume Zoom MDCT scanner (Siemens Medical Solutions, Andover, MA ).
Then, two radiologists working independently assessed the degree of colonic distension subjectively on a Vitrea 2 workstation (Vital Images, Plymouth, MN) for each of six colon segments, and graded the distension on a four-point scale, with 0 representing a collapsed segment, and 3 representing optimal distension.
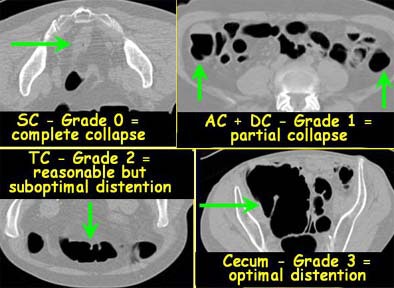 |
| Each colon segment was rated for distension on a scale of 0 (collapsed) to 3 (optimal distension). Images courtesy of Dr. Riccardo Iannaccone. |
Pain and acceptance
"The analysis was performed for both prone and supine positions, as well as for the combined positions," Iannaccone said. "In addition, we tried to evaluate the patients' level of pain and level of acceptance of the procedure. All (132) patients completed the first (questionnaire) right after (VC) to assess the presence of abdominal discomfort and pain, from none (0) to severe (4), during the procedure and their willingness to undergo (VC) in the future, (by answering) a very simple question with a very simple answer: yes, no, don't know."
Finally, to gauge the stability of patients' responses over time, the patients were asked to return the same questionnaire again 24 hours after the exam. In all, 131/162 (80.9%) of patients returned the second questionnaire, Iannaccone said, noting a high level of overall agreement (k=0.81) between the immediate postexam responses and those taken 24 hours later.
Distension
The combined results for prone and supine imaging showed no statistically significant difference in adequacy of distension among the three patient groups (p<0.05). Still, there were slight differences among them. Glucagon slightly improved overall distension in the cecum and the ascending colon compared to placebo, Iannaccone said. Buscopan slightly improved overall distension in the ascending colon, transverse colon, and descending colon compared to placebo. And interestingly, the placebo patients had slightly better distension in the sigmoid colon than the Buscopan group, he said.
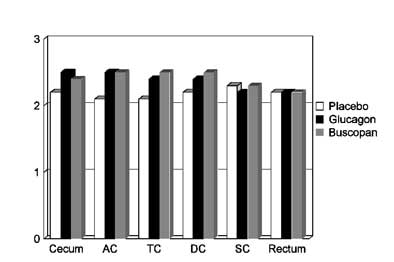 |
| On a scale of 0 (collapsed) to 3 (optimal distension), chart depicts subjectively rated differences in colonic distension between placebo (group 1), Buscopan (group 2), and glucagon (group 3) patients for each of six colon segments: cecum, ascending colon (AC), transverse colon (TC), descending colon (DC), sigmoid colon (SC), and rectum. None of the differences in distension reached statistical significance. Chart courtesy of Dr. Riccardo Iannaccone. |
Pain and willingness
In contrast to the inconclusive distension results, marked differences were noted between the groups in terms of pain and discomfort. To wit: the placebo patients reported significantly higher discomfort and pain (mean, 2.8 on a scale of 1-4) compared with those who received either glucagon (mean 1.6) or Buscopan (mean 1.5) (p<0.5).
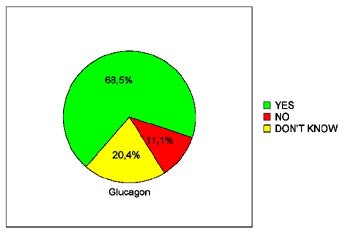
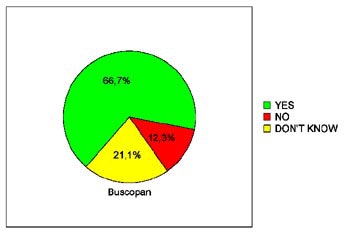
In terms of patients' willingness to undergo virtual colonoscopy again in the future, just 39.3% of the placebo patients were willing to do so, compared to 68% of the glucagon patients and 66.7% of the Buscopan patients. The differences between the placebo and either drug achieved statistical significance (p< 0.05), Iannaccone said.
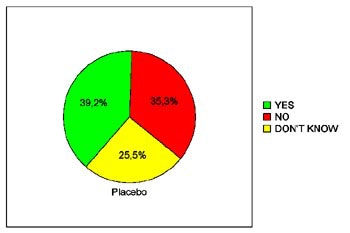 |
| Pie charts demonstrate patients' expressed willingness to undergo virtual colonoscopy screening again in the future after a virtual exam using an intravenous saline placebo (top), 1 mg IV glucagon (below), or 20 mg IV Buscopan (bottom) as an antiperistaltic agent. The differences between those who received the placebo and either drug were statistically significant. Charts courtesy of Dr. Riccardo Iannaccone. |
 |
 |
"Although glucagon and Buscopan can improve the adequacy of colonic distension in specific segments, we were unable in our study to demonstrate a significant improvement in the degree of distension with use of (either) of these drugs, Iannaccone concluded. "However, glucagon and Buscopan are essential to reduce patient discomfort and pain during (VC). Therefore, we recommend that antiperistaltic drugs should always be administered prior to CT colonography in order to increase patient acceptance."
By Eric Barnes
AuntMinnie.com staff writer
August 30, 2004
Related Reading
Jury deadlocks on use of Buscopan in virtual colonoscopy, September 18, 2004
Virtual colonoscopy techniques evolve with technology, July 25, 2003
Imaging and imaging-related drugs: A pharmacist's perspective, October 8, 2002
Copyright © 2004AuntMinnie.com