Stroke is a big-time killer -- the third leading cause of death and the number-one cause of disability in the U.S. A third of the approximately 1,200 strokes that occur each day in the U.S. are fatal, and another third permanently disabling, according to National Institutes of Health statistics.
These days, MR and CT are helping to reduce the devastation of acute stroke by playing an important triage role: separating the patients likely to benefit from antiplatelet or thrombolytic therapy from those in whom intervention would only heighten the risk of injury.
Brain tissue at risk but not yet dead can be identified by pairing diffusion and perfusion MRI, or by means of quantitative perfusion CT imaging. Add vascular mapping with CT or MR, and the brain's status and blood supply can be assessed in real time, and appropriate intervention determined.
Which modality does this triage job best? The short answer is that both MR and CT are excellent diagnostic tools, according to Dr. Jay Cinnamon, director of neuroradiology and associate professor of radiology at the Emory University School of Medicine in Atlanta.
The longer answer says the best modality depends on a lot of factors -- on the clinical question and patient presentation, for example, on available technology and the technologists. It even depends on the practice workflow, practice style, and the art of the image, Cinnamon said.
Stroke considerations
Stroke, Cinnamon explained at the 2004 Symposium on Multidetector-Row CT in San Francisco, is a cerebrovascular accident characterized by acute onset of neurological deficit and altered consciousness. It can result from a number of pathogenic mechanisms, including arterial or venous infarction, and parenchymal or subarachnoid hemorrhage.
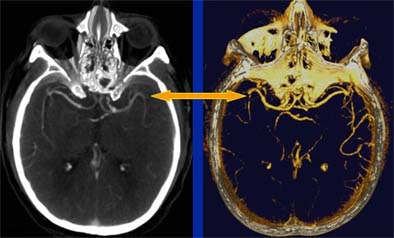 |
| Head CT seen in slab MIP (maximum intensity projection) view (left), and volume-rendered view (right) depict M2 artery occlusion. Images courtesy of Dr. Jay Cinnamon. |
"Stroke is not an infarct, though strokes can be caused by infarcts, and they may be one of the leading etiologies for stroke," he said. And because infarcts are the main focus of imaging (they are responsible for ischemic strokes and more than two-thirds of all stroke cases), they would also be the focus of his talk, Cinnamon said.
Four premises offer a good diagnostic starting point, he said. The first is that stroke imaging and diagnosis is a complex undertaking. Anatomic and physiological considerations are key to getting it right. Even a routine noncontrast CT scan holds a lot of information, Cinnamon explained, and it's important to get everything possible from it. Ask yourself what caused the neurological deficit, what caused the imaging findings, and how the patient is doing, he said.
For example, if a noncontrast CT within three hours of onset shows no hemorrhage but lucency in no more than a third of the area of the middle cerebral artery (MCA), the patient could be a candidate for tissue plasminogen activator (tPA). If more than a third of the MCA area is lucent and there's too much damage, then reperfusion is not worth the trouble.
"There are benefits of giving tPA as long as patients qualify, but there are also some risks that patients can undergo hemorrhagic transformation," he said. Still, in the brain, correlating pathology to the presentation can be a daunting task.
Premise two is that silent brain does not equal dead brain.
"We know what normal perfusion of the brain is, we know when we reach electrical silence, and we know when we get to cell death," Cinnamon said. "If you're above the threshold area, you have electrical silence -- the equivalent of acute neurological deficit -- but the brain is not yet dead. And this whole dynamic of ischemia and perfusion plays into how the patient is going to do, and what you can do to intervene."
Premise three is that even the most skilled clinicians can't always reliably localize the infarct and the site of vascular disease, he stated. Even if they're fortunate enough to localize the infarct within the brain, they cannot always identify the pathogenic mechanism of the disease.
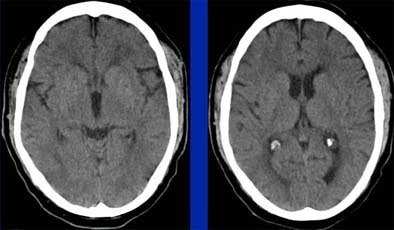 |
| Even in a patient presenting with acute right hemiparesis, routine noncontrast CT can be normal. Images courtesy of Dr. Jay Cinnamon. |
"A small lacunar infarction in the corpus striatum ... can manifest as a sensory deficit, whereas the same-sized infarct 1 cm more anteriorly, in the internal capsule, will leave the patient with a hemiparesis," Cinnamon wrote in an accompanying discussion. "Such high-order architectural detail is unique among the organ systems .... If different types of lesions can lead to similar clinical scenarios, as long as they share a common locus in the CNS, then imaging can play a valuable role in identifying and characterizing the process in each individual case."
The last premise is that different clinicians may want to know different information, Cinnamon said. "There are all sorts of ways to treat stroke, so any diagnostic study is only as good as its ability to answer the clinician's questions. You must communicate with your clinician to know what's important to them, then you can tailor the examination to get the answers."
"We want to rule out hemorrhage, and rule in infarct if we can. We want to identify dead brain, ischemic brain, and penumbra that's potentially salvageable," he said. "We want to identify the site of disease in the arterial tree, and we want to identify a possible pathogenetic mechanism that led to this infarct, this cerebrovascular accident."
Ischemic penumbra
MR and CT can both define ischemic brain tissue at risk for infarction but still living, and therefore potentially salvageable, Cinnamon said. "When you have ischemic brain that's not yet dead, the brain can react with its autoregulatory mechanisms to dilate the capillaries," he said. "It's only when the brain dies that those autoregulatory mechanisms no longer work."
In MR, diffusion-weighted imaging is used to evaluate the "perfusion/diffusion mismatch," or the correlation between the diffusion abnormality and the perfusion abnormality. Once the perfusion/diffusion deficits match, the tissue has presumably died and cannot be salvaged. Conversely, parenchyma that shows a perfusion deficit but normal diffusion can be considered ischemic penumbra and potentially salvageable.
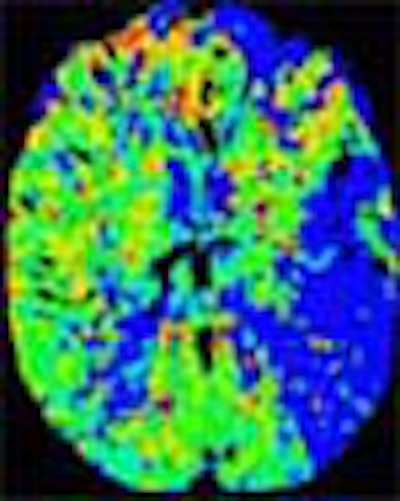
In recent years, researchers have performed CT studies that demonstrate the same information. Initial-phase CT will show ischemic penumbra as a region of normal or increased cerebral blood volume (> 2.5 ml per 100 g of brain tissue) in the region, combined with a decrease in CBF of more than 34%, he said. Once the tissue dies, CBV will also decrease.
"So decreased CBV is dead brain, whereas normal or increased CBV in the setting of decreased CBF is penumbra," he explained. Parametric color maps based on CBV, CBF, and mean transit time (MTT) can be used to illustrate the penumbra.
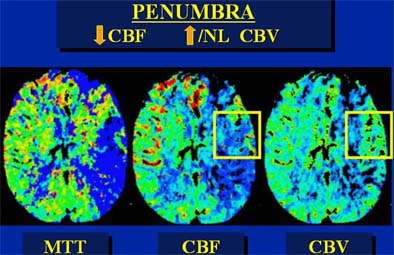 |
| In an elderly male patient, parametric color maps from perfusion CT show dramatic ischemia in the left hemisphere. In the area of the box, cerebral blood flow (CBF) is decreased (> 34%), cerebral blood volume (CBV) is increased (> 2.5 mL per 100 g of brain tissue), explaining the long mean transit time (MTT), and indicating potentially salvageable brain tissue. However, in the region below the box, substantial matching of blue areas (CBF and CBV) indicates infarcted tissue that is not salvageable. Images courtesy of Dr. Jay Cinnamon. |
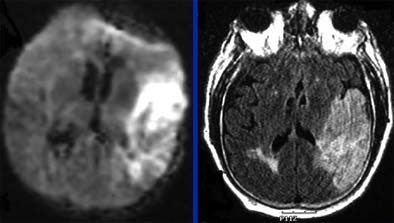 |
| Follow-up MRI (left, DWI or diffusion-weighted imaging; right, FLAIR technique) in the same elderly male patient shows that area of salvageable penumbra seen in initial CT was not saved, and the entire area has become infarcted. Questions relating to the patient's history of gastrointestinal bleeding led to initial decision to forgo tPA. Diffusion-weighted MRI identifies ischemic but living brain parenchyma at risk for infarction by assesssing the correlation between the diffusion abnormality and the MR perfusion abnormality. Images courtesy of Dr. Jay Cinnamon. |
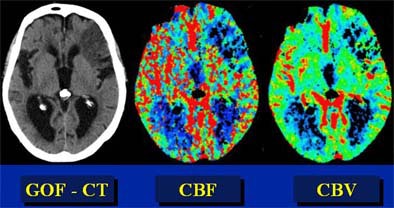 |
| In this patient, "good old-fashioned" (GOF) head CT (left) depicts acute infarction in the left frontal lobe. Perfusion imaging was performed to assess the presence of penumbra. However, parametric color mapping shows substantially matched CBF and CBV, indicating infarcted tissue that is not salvageable. Images courtesy of Dr. Jay Cinnamon. |
Questions and imaging goals
To find the right answers, the radiologist needs to know the clinical questions and the goals of the imaging study, Cinnamon stated.
"Is there hemorrhage? Do I need to identify the infarction as soon as possible? Do I need a vascular map? Do I want to evaluate the penumbra? This is what you have to answer with your clinicians -- what is the information I need to get from my practice?" he said. Sometimes the only question is whether there is hemorrhage. "But sometimes I want to see infarct right from the emergency room."
At that point, the choice of MR versus CT can depend as much on practice style and capabilities as on the clinical question. The radiologist needs to ask himself what kind of environment he is practicing in. Perhaps it is a conservative one, where the only clinical question for the ER docs is whether there is hemorrhage.
"Ask yourself, 'Am I working in an accelerated environment where we want to get the workups done for the patient as quickly as possible, even though we're not going to be poised to act as with interventional therapy?' In which case you may say, 'Let's do a perfusion study or diffusion study,' or 'Let's look at a vascular map at the same time.'"
A moderately aggressive strategy might involve MRI with diffusion-weighted imaging (DWI) to identify the infarction soon after the onset of symptoms, rather than waiting for follow-up CT to define the area of infarction, Cinnamon said.
The accelerated environment can offer important advantages in patient management. For example, most lacunar infarctions are due to spontaneous thrombosis of a perforator artery, calling for antiplatelet therapy, Cinnamon explained in an accompanying abstract. But in a minority of cases, the infarction may be due to large vessel disease such as the M1 branch of the MCA, which calls for anticoagulation therapy.
Perhaps the work environment is very aggressive, with stroke neurologists and interventionists poised to do everything possible for the stroke patient.
In this scenario, "thrombolytic agents, neuroprotective agents, and hemodynamic measures are all fine-tuned and can be tailored to the treatment of each individual patient," Cinnamon wrote.
"This is where all the stops get pulled out as quickly as possible. Infarcted tissue is identified way before it declares itself on routine CT imaging. The penumbra -- i.e., ischemic but potentially salvageable tissue -- is recognized as such. A full vascular map from aortic arch through the circle of Willis is generated. And it all happens stat because time equals brain, and the clinical decision-making is done real-time."
MR versus CT
CT's principle advantages are speed and availability. CT is faster and much more patient-friendly than MRI, Cinnamon said. A comprehensive evaluation including noncontrast CT, a perfusion study, and CTA takes about 10 minutes, and "can be called a screening test and diagnostic test, potentially (avoiding) a catheter angiogram study," he explained. Following acquisition, streamlined CT perfusion postprocessing protocols are fast and easy to perform.
And CT is still the gold standard for ruling out hemorrhage, though some MR techniques such as FLAIR and gradient echo have also been shown high sensitivity for hemorrhage, Cinnamon stated.
CT's disadvantages include the need for iodinated contrast and its inherent risks. Technologist training is also an issue; the tertiary stroke protocol requires training that not all CT technologists on overnight duty may have. Moreover, CT offers a limited field of view, and its sensitivity is limited in the brain stem.
Illustrating his point, Cinnamon showed a slide in which small infarcts in the cerebellum were barely visible on CT. "Unless you're specifically tuned into this, you're not going to see them. So if you're expecting brain stem or cerebellar infarctions, my personal preference is to go the MR route."
Another advantage of MR is that diffusion-weighted imaging is completely noninvasive. "You get a complete field of view in the sense that you can look at the entire brain rather than just 3-4 cm of brain," Cinnamon said, noting that DWI abnormalities occur within minutes of infarction, and long before pathological changes can be detected on CT.
"The brain stem and posterior fossa have limited visibility (in CT)," he said. "The advantage of MRI is that you can see infarcts in this area."
Contrast-enhanced 3D MRA can be just as fast as CTA, although scan times are significantly longer if the MR is used with routine time-of-flight imaging, a technique that can lead to suboptimal visualization of the aortic arch.
A disadvantage of MRI is that it's not patient-friendly, he stated. MRI can provide a claustrophobic experience -- less than optimal for stroke patients who may be agitated. In terms of workflow, MRI is less available in general, and typically not available 24/7.
"(MR) perfusion imaging is still kind of clumsy to do," he noted. "It can be done, but it requires a lot of training." Vascular maps in MRI are typically for screening, and additional evaluation is generally needed to examine suspected stenoses, for example.
MRI has a longer overall scan time than CT (20-30 minutes), he said, and there are consent and safety issues related to metal implants, pacemakers, etc.
Different strokes
In the final analysis, the best modality for stroke imaging might come down to factors not directly related to the modality.
"Know the strengths and weaknesses of your technology, know what's available, know your personnel and what their skill set is, and know your processes and workflow issues," Cinnamon said. It's important to know the other radiologists in your practice and what their skills are in terms of reading the studies.
"The most important thing for the radiologist to know is what the clinician is asking," he said. "Once you know the questions, you can go get the answers. And then choose your flavor: CT or MRI. This is all about the art of technology, and in my opinion these are both very good tools. Understand what your clinicians need to know, and you'll be able to build the right route based on strong lines of communication with them."
By Eric Barnes
AuntMinnie.com staff writer
August 25, 2004
Related Reading
Cerebral microbleeds not linked to cardiovascular risk factors, July 27, 2004
DWI proves best of seven in subacute stroke imaging, May 17, 2004
Short MRI exam extends stroke tPA window, with good results, April 30, 2004
Perfusion CT predicts, measures, thrombolysis benefit after acute stroke, December 24, 2003
Perfusion CT offers speedy access, but MRI gives the "big" picture, February 19, 2003
Copyright © 2004 AuntMinnie.com