That more image details would be visible with additional CT detectors seems logical enough. But real-world examples have been sparse, and clinical studies comparing four scanner generations side by side are rare birds indeed.
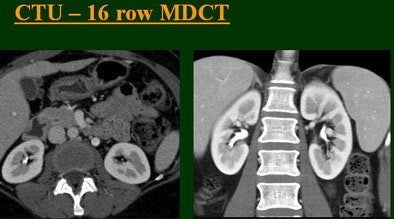
At the 2004 RSNA meeting in Chicago, Dr. Vassilios Raptopoulos from Massachusetts General Hospital and Harvard Medical School in Boston evaluated single-, four-, eight-, and 16-row detector scanners in 200 patients using a single-acquisition biphasic CT urography (CTU) protocol. As the number of detector rows increased, visualization -- especially of the calyces -- improved significantly.
In a subsequent RSNA presentation, Dr. Terri Vrtiska from the Mayo Clinic in Rochester, MN, showed off the diagnostic heft of her group's new 64-slice machine, also in a split-bolus CTU protocol study. The Mayo study represents the fifth scanner generation described in this article to use a split-bolus single-acquisition CTU technique. And in a separate study, Vrtiska's group found better results for the new machine in a separate side-by-side slice comparison, as Raptopoulos' group concluded.
Raptopoulos said that for most patients a single biphasic split-bolus CT urography scan -- combining the corticomedullary and excretory phases -- provides most of the information available in a higher-dose two-series acquisition. Except in a few situations requiring a very detailed look at contrast dynamics in two separate acquisitions, the single-scan approach is robust enough for CTU and general abdominal CT as well. And the split-bolus technique seems to produce better results every time the group gets a new scanner, he said.
"As we've been scanning through the kidneys, we've been noticing that there has been an increase in the quality images as we go from (single-) to four-, eight-, and 16-row detector scans," Raptopoulos said. "Our purpose, then, was to assess the quality of CTU with progressively improving CT technology from (single-) to 4-, 8-, and 16-row scanners, using a split-injection single scanning technique."
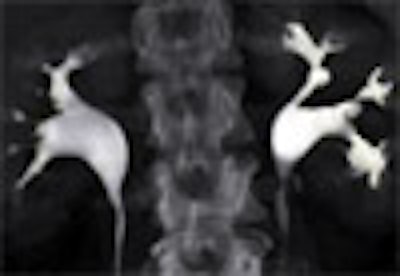
 |
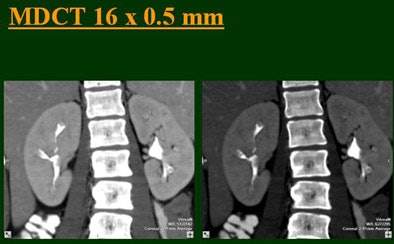
| Top to bottom: Split-bolus CTU improves significantly using a nearly identical protocol but with the addition of more detector rows, from single- (spiral), four-, eight-, and 16-row scanners. All images courtesy of Dr. Vassilios Raptopoulos. |
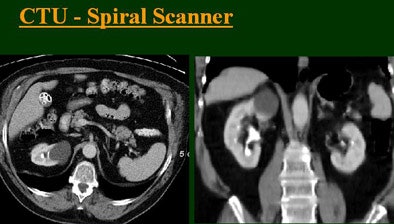
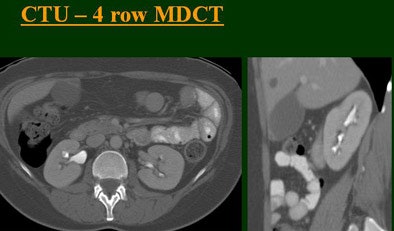 |
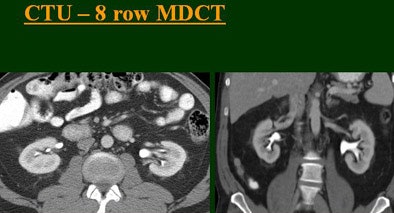 |
 |
 |
The group examined 200 patients referred for CTU over an eight-month period, and divided them into four groups of 50 each that underwent either single-, four-, eight-, or 16-row CTU (The first three scanners were from GE Healthcare, Chalfont St. Giles, U.K.; the 16-row machine was from Toshiba America Medical Systems, Tustin, CA.)
The patients were prepped with about 4 cups of water 30-60 minutes before the study. A noncontrast series was obtained first, followed by contrast-enhanced scanning using the split-bolus technique. The patients received 50 mL of nonionic IV contrast at 2.5 mL/sec without being scanned, then two minutes later an additional 100 cc of contrast was administered at 2.5 mL/sec, and images were acquired from the diaphragm to the symphysis 60 seconds after the second injection.
The single-row scanner required two breath-holds; the other scanners reduced scan time from about 20 seconds (four-slice) down to seven seconds (16-slice). Collimation for the single-row scanner was 5 mm, with 3-mm overlapping reconstructions. Collimation for the four-, eight-, and 16-row scanners was 2.5 mm, 1.5 mm, and 0.5 mm, respectively, with the patients scanned in a prone position.
Visualization of the calyces was evaluated subjectively on a scale of 1 to 5, with 1 representing no visible architecture and 5 representing sharply cupped calyces. The filling of the upper and lower ureters was also scored: 1 for no filling, 3 for half filled, and 5 for completely filled.
Based on multiplanar reformatted (MPR) images, there was significant upscale improvement in calyceal detail from the isotropic images provided by single-detector scanning (median score 2) to four- (median score 3), eight- (median score 4), and 16-detector scanning, Raptopoulos said. On MPR images the calyceal were 1, 2, 3, and 5, respectively. The median ureteral filling was 4 for the upper and left-lower ureters, and 3 for the right-lower ureters.
When the ureters were filled, "we counted in how many slices we saw ureters," he said. "The ureters did fairly well, (though) we had a problem .... with the right-lower ureters, which were filled a little less consistently than the rest of the ureters."
"We're using this technique of biphasic injections not only in CTU, but in general abdominal scanning in trauma, in renal CT, and we can see the renal vessels quite well in relation to the collecting systems," Raptopoulos said. "Combined excretory- and parenchymal-phase CTU is a practical technique, allowing a decrease in patient scanning," he said. "Sixteen-row CTU with the patient in a prone position significantly improves the quality of the exam."
Even better at 64
In a special "hot topic" presentation during the same RSNA session, Dr. Terry Vrtiska from the Mayo Clinic in Rochester, MN, presented her group's initial CTU results, obtained in the first three months of using the group's new Somatom Sensation 64-slice CT scanner (Siemens Medical Solutions, Andover, MA).
A single breath-hold split-bolus technique was designed to maximize acquisition speed while yielding isotropic (0.4-mm) resolution, Vrtiska said. The scans were performed in 12 patients (seven men and five women, ages 30-78) with a history of hematuria (n = 7) or transitional cell carcinoma (n = 5).
First, 70 cc of nonionic iodinated contrast was administered at 4 cc/sec (Omnipaque 300, GE Healthcare Bio-Sciences), followed immediately by 70 cc of normal saline at 4 cc/sec, Vrtiska said. After a six-minute delay, ureteral compression was applied above the anterior superior iliac spine. Then an additional 70 cc of contrast was injected at 4 cc/sec, followed by another 70-second delay. Then the ureteral compression was released, the patient was asked to cough twice, and an abdominopelvic (AP) CT was obtained (including two automated axial reconstructions: 3-mm slice thickness/3-mm intervals and 1-mm slice thickness/0.8-mm intervals).
Coronal maximum intensity projection (MIP) images were automatically created from the operator's console (2-mm thickness/1.8-mm intervals). The split-bolus technique only required a single contrast-enhanced acquisition of the abdomen and pelvis, where the first injection was in the collecting system, and the second in the renal parenchyma.
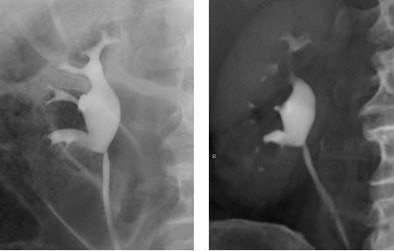 |
| Retrograde pyelogram versus IVU. Left, normal right retrograde pyelogram. Right, single volume-rendered CT image obtained during 64-channel CTU. Images courtesy of Dr. Terri Vrtiska, Mayo Clinic, Rochester, MN. |
In addition, CT scanned projection radiographs (SPRs) (300 mAs, 80 kVp) with compression as well as ureteral decompression SPRs in the supine and the prone position were obtained after the AP CT scan. At least three optimized volume-rendered (VR) images were immediately obtained by the radiologist using the 1-mm slice thickness/0.8-mm interval dataset and sent to the PACS.
"While the initial experience is promising, ongoing research using 64-channel technology is being done in order to optimize the contrast dose, injection rates, injection times, and reconstruction algorithms," Vrtiska told AuntMinnie.com in an e-mail.
The left and right ureters were divided into four segments each for evaluation -- a total of 96 segments in 12 patients. In all, 90% of the segments were opacified, including all the kidneys and pelvi, she stated. One proximal ureter was not opacified in a patient with a left ureteropelvic junction (UPJ) obstruction. Three large ureters remained unopacified, but two of the three could be seen on either the prone or decompression scout view, she said. And while 10 distal ureter segments were not opacified, eight of the 10 were opacified on either the prone or decompression scout views.
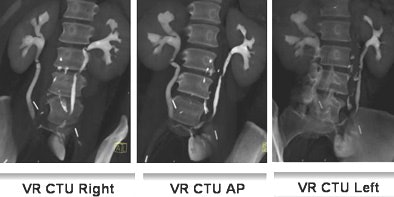 |
| Fifty-three-year-old woman post cystectomy. Three VR CTU images (left to right: right oblique, AP, and left oblique) were created to depict the ileal neobladder reconstruction. Images courtesy of Dr. Terri Vrtiska, Mayo Clinic, Rochester, MN. |
"A total of 92 of the 96 segments were opacified, and if the patient with the left UPJ obstruction had been excluded, since it wasn't possible to opacify that, it would have been 92 of the 93 segments," Vrtiska said.
The findings included medullary sponge kidney in three patients, UPJ obstruction in one, bladder transitional cell carcinoma in one, postoperative urinary tract reconstruction in two, upper tract indeterminate filling defect in two, and normal results in three patients. Two of three ureters that were not opacified in VR could be seen in either the prone or decompression scout views.
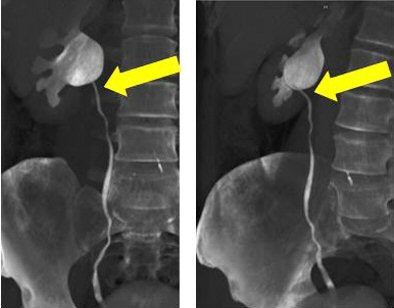 |
| Volume-rendered CT images obtained during 64-channel CTU with interactive display of the collecting system and the relation of the right renal pelvis to the proximal ureter with interactive manipulation of the isotropic dataset. Images courtesy of Dr. Terri Vrtiska, Mayo Clinic, Rochester, MN. |
The single-breath-hold protocol, combined with MIP and VRT postprocessing techniques, provides 0.4-mm isotropic CT, enabling the visualization of urothelial pathology routinely seen on traditional IV urography, she concluded.
A separate study Vrtiska presented at the RSNA meeting used a phantom with simulated contrast-filled ureters to compare the spatial resolution of their 64-slice CTU protocol to published protocols for other MDCT scanners, including four- and 16-slice scanners, as well as computed radiography images, which were reviewed by two gastrointestinal radiologists who scored each filling defect.
The 64-slice scanner, using 0.6-mm collimation and 0.3-mm reconstructions, was able to identify the greatest number of filling defects -- identical to computed radiography -- and was the only scanner/protocol to identify the smallest filling defects, the group concluded. "The resolution of 16-(slice) MDCT (1.25 mm/0.6 mm) was slightly less than 64-(slice) MDCT, and required longer scan times. The 4-(slice) MDCT was the most limited in resolution," Vrtiska and colleagues wrote in their abstract.
By Eric Barnes
AuntMinnie.com staff writer
March 18, 2005
Related Reading
CT gains ground in urology, June 18, 2004
Principles of Genitourinary Radiology, March 11, 2004
Multiphasic MDCT with contrast boosts liver, urography results March 1, 2004
Copyright © 2005 AuntMinnie.com




















