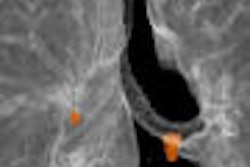
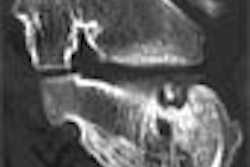
Biomedical nanotechnology firm pSivida of Perth, Australia, announced the final results from its phase IIa clinical trial with BrachySil, a new brachytherapy treatment for inoperable primary liver cancer.
The trial was conducted at the Singapore General Hospital on eight patients with advanced liver cancer who were evaluated at three and six months following treatment. The micron-sized, nanostructured silicon phophorus-32 particle was found to be safe, effective, and also reduced the size of tumors based on CT scans, according to the company.
A multicenter phase IIb dose-profiling study is scheduled to begin later in 2005.
By AuntMinnie.com staff writers
June 21, 2005
Related Reading
BrachySil data to debut at ESTRO, May 4, 2005
Copyright © 2005 AuntMinnie.com