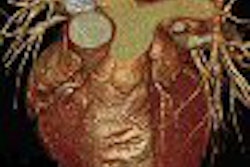
Toshiba America Medical Systems of Tustin, CA, said it has received Food and Drug Administration clearance for its Aquilion LB (large bore) CT scanner.
The firm reported that FDA clearance was granted in March, and that it has now begun commercial shipments of the units in the U.S. The device has a 90-cm bore opening and an acquired field-of-view of 70 cm, according to Toshiba. It also features the company's proprietary QuantumPlus detector, which can provide three different slice-width acquisitions, enabling it to acquire isotropic images, Toshiba said.
By AuntMinnie.com staff writers
September 29, 2005
Related Reading
Toshiba donates $1 million for hurricane relief, September 5, 2005
Toshiba debuts Famio, August 22, 2005
Toshiba nets 64-slice CT install, August 16, 2005
Toshiba debuts Infinix CB-I, August 12, 2005
Toshiba selects Cooper for CT slot, July 28, 2005
Copyright © 2005 AuntMinnie.com