SAN FRANCISCO - Hundreds of family physicians jammed a lecture room at the Moscone Center Saturday to hear about controversies in colorectal cancer screening. As the session began, participants were warned that the fire marshal was keeping a close eye on the heavily attended American Academy of Family Physicians (AAFP) meeting, and would shut down the session if people were found standing in the doors and exitways.
But there was no fire, and for that matter, not a spark of controversy surrounding the day's discussions of virtual colonoscopy for colorectal cancer screening.
Dr. John Pope, a professor of clinical family medicine at Louisiana State University in Shreveport who performs and teaches optical colonoscopy to physicians, spoke positively of VC's potential as a future screening tool. But he found no room for it in any current screening scenarios.
For better or worse, such is the mainstream view among gastroenterology professionals, of course, though the persistent exclusion is enough to inflame some VC proponents, who believe the evidence already supports its use in a number of indications, such as after failed colonoscopy, and that VC's proper role will ultimately be far broader.
Audience members, who peppered the speaker with dozens of questions about difficult screening scenarios, didn't mention VC, though five currently approved exams for colorectal cancer screening -- optical colonoscopy, flexible sigmoidoscopy, fecal occult blood test (FOBT), double-contrast barium enema, and fecal immunochemical test (FIT) -- offered plenty of controversy on their own. What to do, for example, with the patient who has a positive FOBT but refuses to undergo invasive colonoscopy?
Each method approved for reimbursement under current U.S. screening guidelines comes with its own strengths and limitations, of course. On one hand, FOBT is inexpensive, noninvasive, easy to administer, and well-studied. It is the only colorectal screening exam to show a colorectal cancer (but not general) mortality benefit, Pope noted. On the other hand, it suffers from extremely low sensitivity of less than 5%.
According to American Cancer Society guidelines, men and women at average risk should follow one of five approved screening options:
- Yearly FOBT or FIT
- Flexible sigmoidoscopy every five years
- Yearly FOBT plus flexible sigmoidoscopy every five years
(Of these, the ACS prefers FOBT or FIT every year plus flexible sigmoidoscopy every five years.) - Double contrast barium enema every five years
- Colonoscopy every 10 years
Optical colonoscopy, or the "default gold standard," as Pope called it, does an excellent job of examining the colon. But even in experienced hands it misses 5% to 12% of advanced lesions, and carries a small risk of perforation, Pope said, adding that it took virtual colonoscopy to uncover optical colonoscopy's limitations.
Flexible sigmoidoscopy is far less invasive and can be combined with FOBT for effective screening, he said, but inasmuch as it cannot examine the entire colon, it misses distal cancers.
The biggest problem is still compliance, Pope said. Despite its being the second biggest cause of cancer deaths, colorectal cancer simply hasn't sparked the rush for screening that has accompanied other cancers. It is particularly unfortunate, he said, because the adenoma-carcinoma sequence offers a generous amount of time -- a decade or more -- to remove most advanced polyps before they become invasive cancers. Less than 40% of the eligible population has undergone colorectal screening of any kind, he said.
"For some reason, colon cancer just doesn't motivate people for getting checked for it," Pope said, whether due to the invasiveness and the discomfort of screening, the embarrassment, or some other factor. In fact, a physician's advice is the only factor that has been shown to increase compliance in any meaningful way, he said.
"The thing that seems to matter the most is when the doctor recommends it," Pope said. It means that the family physician has a critical role to play in saving lives, he said.
Which tests to recommend and in which combinations formed the brunt of the discussion. Flexible sigmoidoscopy still has a strong role despite well-known limitations, Pope said.
"(Flexible sigmoidoscopy) is a very useful test because it allows us to see approximately 30% to 50% of the colon," he said. "We know that colorectal cancers are spread out pretty much evenly throughout the colon and rectum. And we know that anywhere from 45% to even 60% of our colorectal cancers are located within the region touched by the sigmoidosocope -- in other words distal to the splenic flexure."
Moreover, good data show that many of the more proximal cancers are accompanied by a precursor lesion that is accessible to the sigmoidsocope, he said. So even if the proximal cancer is missed, the test finds a precursor lesion that leads to further examination of the patient.
"If you do the math on (flexible sigmoidoscopy), you come up with a potential test finding 75% of our adenomatous polyps and colon cancers," Pope said. "I can't call a screening test that has that much potential, is relatively inexpensive, is relatively safe, is readily performed, and can be learned and performed by primary care physicians a bad thing. So yes, I incorporate that."
"I have patients that will not do colonoscopy," he said. "If I'm able to get them to agree to do annual fecal occult blood testing, then I feel I'm doing something positive."
At any rate, Pope said, doctors must steer clear of the notion that the default exam, colonoscopy, is a perfect test. It was the development of virtual colonoscopy, which sees the colon in a completely different way, that showed researchers that conventional colonoscopy was less accurate than previously thought.
Before VC, studies on colonoscopy miss rates were always based on repeat colonoscopy as a backup exam, he said. Colonoscopy is known to miss lesions on the proximal side of folds and in other blind spots in the colon.
"If you didn't see (the lesion) because it was in a technically difficult spot, guess what? You're not necessarily going to see it on repeat colonoscopy," he said.
"My point is this: There are no 100% tests, and anytime you're talking about screening patients, you should counsel them in that vein," Pope said. "We're talking about tests that can reduce your risk of colorectal cancer, not eliminate your risk of colon cancer."
At the same time, some screening tests have been shown to be cost-effective, he said, including the combination of annual FOBT and flexible sigmoidoscopy every five years, or colonoscopy every 10 years, Pope said.
"Just remember that any cost-effectiveness test is based on a series of assumptions," he said. "If your assumption is that it reduces mortality but you haven't proved that, you'd better be careful about calling it a cost-effective test."
Another available option is stool DNA testing, specifically the PreGen-Plus test by Exact Science, he said. The test looks at several genetic mutations, with both positive and negative effects, he said. Too many kinds of mutations can lower the specificity of a test, though, obviously, each mutation measured increases the sensitivity.
"The fact is we've been a little disappointed with the sensitivity in the trials," he said. It's not the 95% to 98% sensitivity we would have liked to have seen.... Some improvements and modifications are ongoing in stool testing. The problem is separating the human DNA from bacterial DNA, being able to extract enough of it, and then picking the right mutations and identifying enough of those to enhance it."
Using larger stool samples may improve performance of the tests, he said. But DNA has simply not been tested enough to be a recommended option at this time.
One family physician complained that there are gastroenterologists in his community on every corner, like Shell gas stations. The fear is that if the practice recommends any screening test but colonoscopy, there will be legal trouble if the patient should develop colon cancer down the road, the participant said.
"You must consider your community standards," Pope said. "On the comforting side, every national organization holds these five (screening methods) to be acceptable in average-risk population -- cut and dried."
One family practice physician said he believes colonoscopy is far too invasive a screening test for average-risk patients, and for that reason he does not recommend it in the absence of symptoms. Still, he said, effective alternatives were lacking.
Another doctor in the audience said that an asymptomatic patient's insurance carrier had refused to pay for screening colonoscopy even though several family members had been previously diagnosed with hereditary nonpolyposis colorectal cancer (HNPCC). The doctor said he had to lie about rectal bleeding on the insurance form just to get the patient a colonoscopy, and sure enough, the exam revealed an invasive carcinoma.
There is an ongoing problem with some payors who can't see their interests beyond the current financial period, Pope commented. "Sometimes they look at the here and now, and they don't take a long-term approach when they're doing their bean counting," he said.
Prevention
Preventive strategies are a tricky subject, Pope said. Colon cancer is rare in countries where high-fiber, low-fat diets are the norm, but efforts to reduce colon cancer rates in the U.S. with the same approach have yielded unexceptional results. "Maybe we didn't do it soon enough or long enough, or at the right time," he said.
Supplemental calcium, aspirin, and NSAIDs (nonsteroidal antiinflammatory drugs) have been shown to be the best preventive strategies so far, Pope said, but experience with them is still insufficient to recommend them to patients.
New technologies
Among colorectal screening exams being developed for the future, the science of proteomics examines large panels of proteins expressed by a genome. The process can isolate abnormal proteins that may be manifestations of cancer tumors, Pope said.
Another experimental process, surface-enhanced laser desorption and ionization, ionizes particles from a very small tissue sample and looks for tumor markers in their travel characteristics via mass spectroscopy.
Enhanced colonoscopy techniques are helping to assess lesions without the need for histologic examination. New tools include high-magnification colonoscopes, colon dyes and stains, and laser examination of lesions that evaluates the light scatter properties of various colonoscopy findings to assess their potential for harboring cancer.
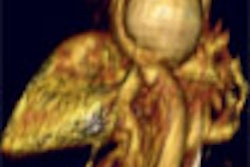
Virtual colonoscopy is likely to impact screening significantly in the near term, Pope said. "There's pretty good data that the sensitivity and specificity for CT colonography (VC) are very good," he said. "The issue is that it doesn't do a great job of looking for small polyps. On the other hand, we're not sure of the clinical relevance or significance of small lesions."
Pope doesn't recommend virtual colonoscopy as part of the screening mix now, despite favorable results compared to other recommended screening tools, because VC is not yet approved by national organizations in the U.S., and because multicenter trial results have shown variable results, Pope said in a later interview with AuntMinnie.com.
"There are still issues of dealing with stool," he said. "Do we need to tag? How much of a prep do we need? If you've got to prep them and they go through all the things you do with a colonoscopy anyway, maybe we're better off with the colonoscopy, where we can remove the lesion."
VC needs more fine-tuning and more trial results to answer the remaining questions, but the technique represents the most important short-term change under way in colorectal cancer screening practice, he said.
By Eric Barnes
AuntMinnie.com staff writer
October 3, 2005
Related Reading
VC caveat: Miles to go before widespread screening, September 23, 2005
Virtual colonoscopy: Ready already for those who know how, April 15, 2005
CMS approves VC after failed colonoscopy in Midwest, August 30, 2005
VC practice thrives in Wisconsin, May 10, 2005
Debate over 2D versus 3D VC reveals subtle differences, January 21, 2005
Copyright © 2005 AuntMinnie.com