Researchers from Johns Hopkins Department of Radiology and Institute of Cell Engineering in Baltimore, MD, have successfully used SPECT/CT to track bone marrow stem cells injected into dogs whose hearts were damaged by heart attack, according to a study published in the September 6 issue of Circulation.
The team -- which includes lead investigator Dr. Dara Kraitchman, Ph.D.; Jeff Bulte, Ph.D.; Wesley Gilson, Ph.D.; Mark Pittenger, Ph.D.; W. Paul Segars, Ph.D.; Benjamin Tsui, Ph.D.; Dr. Richard Wahl; Dr. Randell Young; and others -- used GE Healthcare's Millenium VG/Hawkeye and Signa CV/i 1.5-tesla MR scanner to monitor canine mesenchymal stem cell (MSC) migration patterns after the cells were injected into six dogs with acute myocardial infarction (Circulation, September 6, 2005, Vol. 112:6, pp. 1451-1461).
The cells were labeled with indium-111 oxine, chosen for its relatively long half-life of about three days, as well as Berlex Laboratories' MRI contrast agent Magnevist. Scans were taken immediately after injection and at multiple times over the course of seven days. The stem cells were provided by Osiris Therapeutics, a biotechnology firm also in Baltimore that has investigational new drug approval for using stem cells in research of acute myocardial infarction. Bone marrow was harvested from mongrel dogs and the MSCs were expanded in vitro. All the dogs received radiolabeled and MR-labeled MSCs from unmatched donors for the study.
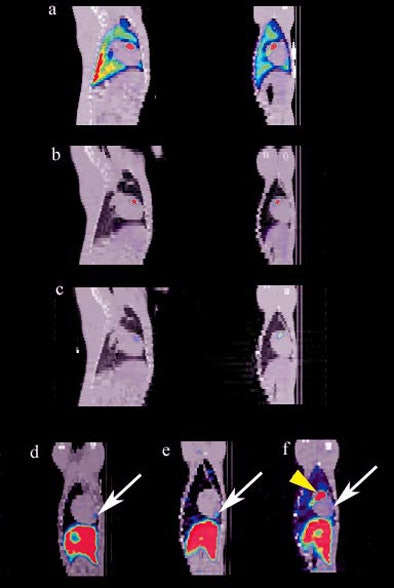
The SPECT/CT images revealed the redistribution of radiolabeled stem cells from their initial landing point in the lungs to the heart, as well as to nontarget organs such as the liver, kidney, and spleen. Since the cells redistributed to other areas of the body, Kraitchman and her colleagues developed specialized reconstruction algorithms to ensure that they were seeing the heart in the images they collected.
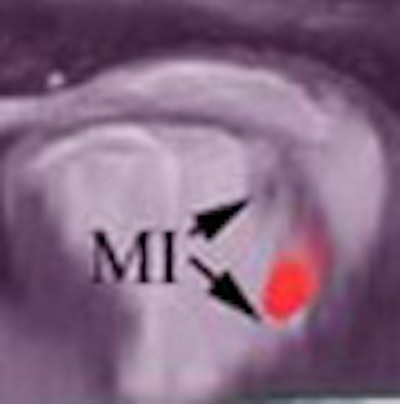
 |
| Combined SPECT/CT images on days 1 (A), 2 (B), and 7 (C) in a dog that demonstrated focal uptake in the anterior midventricular region of the heart. D to F, at the last imaging time point (days 5 to 8), a region of MSC uptake (arrow) is shown in three representative animals. All images courtesy of Kraitchman et al, Johns Hopkins University. |
The team validated their findings by measuring the radiation levels in these tissues postmortem. MRI images did not reveal the cardiac localization of the stem cells, due to its lower sensitivity.
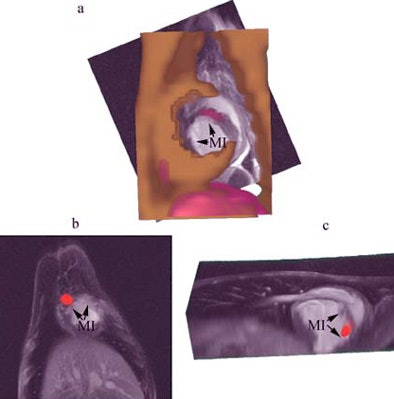 |
| Combined SPECT/CT and MR images of the heart demonstrating focal uptake of indium-111 oxine-labeled MSCs in the peri-infarcted region. A, CT image (gold) combined with MRI (grayscale) and SPECT (red) shows focal uptake in the myocardial infarct (MI) area. B, focal uptake on SPECT (red) in another animal demonstrating localization of the MSCs to the MI in the two views (B, C). |
"SPECT/CT is a good technology for tracking stem cells because SPECT offers high resolution for the cells we're looking for, and CT gives the anatomical picture," Kraitchman said. "MRI is not as effective in tracking the small volume of cells we're monitoring."
The mechanism by which the stem cells homed in on the heart is not completely clear, Kraitchman said. Bone marrow-derived stem cells have receptors that natively attract them to sites of injury for tissue repair similar to inflammatory cells. Exogeneous delivery of enriched quantities of MSCs expands the available pool for homing to the myocardial infarction. The MSCs could have been delivered by nonspecific mechanisms, such as leaky blood vessels that occur during the first few days after ischemic injury, although the researchers believe that it is likely that the continued redistribution of stem cells to the heart after a week was due to active homing rather than vessel leakage.
Kraitchman's team used larger animals than have been used before for radiolabeled cell tracking, and did not open the animals' chest to induce MI, but instead performed a closed-chest left anterior descending coronary artery balloon occlusion using x-ray cardiac catheterization techniques. The team hoped that leaving the animals' chests closed would eliminate the potential confusion about whether the stem cells homing had been dramatically enhanced by the signaling mechanisms created by the chest wound rather than the MI itself.
"The model we used is important," she said. "Our research was done on clinical scanners, with clinical contrast agents already approved for other uses, and could easily be translated to patient studies. Most current clinical trials have had no way of knowing whether the cells actually get to where they're intended to go. Being able to track the cells raises confidence in interpreting outcomes in clinical trials."
As it confirms SPECT/CT's effectiveness in tracking stem cells' distribution patterns, the team's research points to the next step: further studies that address what particular stem cells help particular conditions, appropriate dosing and timing, whether the cells differentiate once they are at the therapy site or remain the same, and whether it's necessary to place the stem cells carefully in the infarction region, or whether they can be injected into tissue that's not infracted and still provide the same benefit.
Researchers and clinicians expect stem cells to offer many therapeutic benefits, including repairing the heart after acute MI; preventing adverse cardiac remodeling after congestive heart failure; preventing or ameliorating cardiotoxicity in breast cancer patients who have received combination therapies such as doxorubicin and trastuzumab; angiogenic therapy in patients with peripheral vascular disease; repairing the brain after stroke; and treating burn patients' wounds. As applications for stem cells increase, tracking their therapeutic use via imaging technology will become routine clinical practice.
"With cell tracking, we can monitor whether a therapeutic dose of stem cells has been delivered and remains in the organ of interest," Kraitchman said. "We can also determine whether the dose is going to nontarget organs and whether there are any potential adverse events or benefits associated with cells in these organs, which allows therapy to be tailored on a patient-by-patient basis. Noninvasive imaging will be fundamental for determining whether positive improvements in organ function from cellular therapy are related to the cells presence or not, as well as which cell type elicits the best response. It will also provide a way to follow organ function over time."
By Kate Madden Yee
AuntMinnie.com contributing writer
October 7, 2005
Related Reading
SPECT/CT pilots prostate brachytherapy in community setting, August 15, 2005
Pinhole SPECT shows promise for arthritis imaging, July 22, 2005
Can SPECT/CT revitalize nuclear medicine? July 7, 2005
MS stem cell therapy in mice can be monitored by MRI, December 2, 2004
Stem cell transplant may help regenerate ischemic myocardial tissue, November 30, 2004
Copyright © 2005 AuntMinnie.com