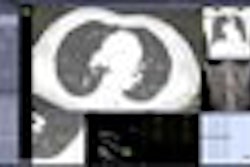
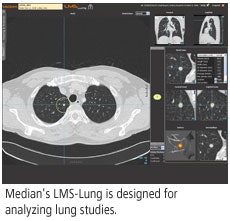
This French developer of computer-aided detection (CAD) software will introduce its Lesions Management Solution (LMS) package, a suite of works-in-progress CAD applications for lung, colon, and liver studies.
LMS applications are designed to provide clinicians with automated tools for image visualization, lesion detection, quantification, volume growth assessment, and automatic reporting on CT scans, according to the company, which is based in Sophia Antipolis. The software is designed to be integrated with PACS workstations installed in a healthcare enterprise.
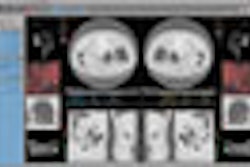
Other applications in the package include LMS-Colon for the analysis of virtual colonoscopy images, and LMS-Liver for quantifying and following hepatic lesions over time on CT scans. Neither LMS-Colon nor LMS-Liver have FDA clearance -- the company expects to file for clearance in the first quarter of 2007.
LMS-Lung and LMS-Colon have already been introduced in Europe, while European introduction of LMS-Liver is expected in the first quarter of 2007.
By Brian Casey
AuntMinnie.com staff writer
November 3, 2006
Copyright © 2006 AuntMinnie.com