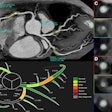
Computer-aided detection software developer EDDA Technology of Princeton Junction, NJ, has received clearance from the U.S. Food and Drug Administration to market its IQQA-Liver application.
The software is an image analysis system that provides diagnostic decision support for physician evaluation of hepatic lesions using multiphase multidetector CT, according to the company.
By AuntMinnie.com staff writers
November 16, 2006
Related Reading
Road to RSNA, EDDA Technology, November 3, 2006
Philips inks EDDA deal, finishes Witt buy, April 28, 2006
Road to RSNA, EDDA Technology, November 17, 2005
EDDA gets FDA nod for chest software, November 22, 2004
Copyright © 2006 AuntMinnie.com