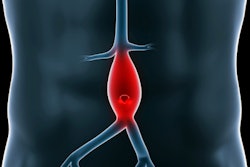
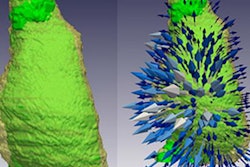
Bordeaux, France-based company Nurea has received U.S. Food and Drug Administration (FDA) clearance for its AI software for measuring aortic diameters on contrast-enhanced and noncontrast CT scans.
PRAEVAorta 2 is designed to support the diagnosis and patient follow-up in aortic aneurysm management, the company said. The software provides an automated reconstruction and analysis of the arterial tree from DICOM images and has the capacity to differentiate the lumen from the thrombus, which enables the extraction of multiple additional criteria not easily accessible for physicians, Nurea noted.
The software has also received the CE mark for use in Europe, the company said.