In the anticipated rush of lung cancer screening subjects to come, automated nodule volume measurement, analysis, matching, and siting will be crucial to workflow and diagnostic accuracy.
Patient management decisions hinge on whether CT-detected nodules are large enough, or have grown enough over time, to warrant biopsy or resection. And there are too many solitary pulmonary nodules (SPNs) to follow manually even now, with most lung scanning relegated to research.
In two recent studies that looked at automated detection software, researchers from the Netherlands examined the accuracy and reproducibility of automated nodule volume measurements in LungCare software (Siemens Medical Solutions, Erlangen, Germany).
Of course, nodule size and location aren't the whole story; growth rate, morphology, solid or nonsolid composition, and other factors alter an SPN's risk of malignancy. But the limitations of commercially available computer-aided detection (CAD) software means that most nodule features still require visual examination by the radiologist.
But a third study, by researchers from California and New Jersey, seeks to automatically measure the solid component of ground-glass nodules containing both solid and nonsolid components.
These issues were explored in three presentations at the 2007 European Congress of Radiology (ECR) in Vienna.
LungCare variability
Manual measurement of solitary pulmonary nodules is too time-consuming to be practical for large patient volumes. And the accuracy of manual measurements, such as those of automated systems, is suspect. But on the whole, small nodules are more likely to be benign, and therefore less likely to be biopsied. At follow-up CT, nodules that haven't grown since the baseline are generally left for future follow-up.
In her ECR presentation, Dr. Anna Leuswald from the University of Groningen in the Netherlands presented her group's study comparing nodule characteristics to the accuracy of automated volume measurements.
The team sought to "assess interobserver variability and analyze the influence of software-generated measurements of pulmonary nodules in a lung cancer screening study," Leuswald said. "We used data from the Nelson study, a Dutch-Belgian randomized control study."
The data included 6,744 baseline scans acquired in 2004 and 2005 using low-dose multidetector-row CT (MDCT). From these data the researchers included all noncalcified nodules 15-500 mm3 (approximately 3-10 mm in diameter) for analysis. Each scan was read independently by two readers, and semiautomated 3D measurements were made with LungCare. The level of agreement was expressed by the absolute values of the relative volume differences (RVDs).
"We would identify a nodule, then click on the nodule, and this would start measurement by the software," Leuswald said. "Location was classified as intraparenchymal or nonintraparenchymal, and nonintraparenchymal nodules were further defined as plural based, juxtavascular, and vessel-attached. Volume measurements were compared between the two readers ... to check interobserver volume variability, and the percentage of complete volume agreement between different subgroups was tested with Chi square tests."
The results showed that 2,993 (66.9%) nodules were intraparenchymal, 765 (17.1%) were pleural-based, 218 (4.9%) were vessel-attached, and 501 (11.2%) were fissure-attached. RVD and the 95% limits of agreement were calculated using the Bland and Altman method.
LungCare versus the radiologists' measurements yielded "complete volume agreement of nodules in 85% all nodules, and a large discrepancy in 4.3% of all nodules," Leuswald said. "Juxtavascular, pleural-based, and fissure-attached nodules had a larger variability."
The 95% limits of agreement of RVD ranged from -10.3% to 11.1% for intraparenchymal nodules, and -18.8% to 20.9% for nonintraparenchymal nodules. The mean RVD was 0.4% ± 5.4 for intraparenchymal nodules, 0.1% ± 9.8% for pleural-based nodules, 0.1% ± 13.8% for vessel-attached nodules, and 0.5% ± 8.7% for fissure-attached nodules.
Broken down by location, measurements were equivalent in 90.1% of intraparenchymal nodules, 81.4% of pleural-based nodules, 72.3% fissure-attached nodules, 67.0% of vessel-attached nodules separately, with significant differences within the group (p < 0.001, Chi square test).
Volume measurements by LungCare software "are reproducible in most nodules, and nonintraparenchymal nodules have a larger variability in software volume than nonintraparenchymal nodules," Leuswald concluded.
Session moderator Dr. Beatrice Feragalli, from the University of Chieti in Italy, asked if this variability would yield too many false positives, and if so, whether increasing the nodule size threshold might be able to compensate for it.
"I think if you use only computer measurements you will definitely have false positives," Leuswald said, "but we have a follow-up mode on the software that allows you to see the baseline and follow-up (nodules) at the same time. I hope this will prevent false positives."
The second LungCare study used the same Nelson database and software to assess the influence of nodule characteristics other than location on automated volume measurements.
In addition to location (intraparenchymal or nonintraparenchymal; pleura-, vessel-, or fissure-attached), Dr. Y. Wang from the University of Groningen and her colleagues assessed the effects of solid nodule morphology (smooth, polylobulated, speculated, or irregular) and size (≤ 50 mm3 or > 50 mm3) on the variability of LungCare-generated volume measurements.
"All scans were performed using low-dose, high-resolution multidetector CT," Wang said.
RVDs were again used to express volume differences, and odds ratios were calculated to independently quantify the effect of morphology, location, and size compared to smooth, intraparenchymal, and small nodules.
In all, 85.5% (3,828/4,477) nodules demonstrated complete volume agreement, and 4.5% (203/4,477) showed small volume differences (RVD range, 0-5%). Another 5.6% (252/4,477) nodules showed moderate (RVD range, 5%-15%) volume disagreement, and 4.3% (194/4,477) showed large volume disagreement (RVD > 15%).
"The odds ratios were 14.7, 4.9, 2.6, 2.4, 2.1, 2.1, and 1.2 for irregular, vessel-attached, spiculated, pleural-based, fissure-attached, polylobulated, and larger nodules, respectively," the authors wrote in their abstract.
Morphology and location can influence the variability of software-generated volume, especially for irregular and vessel-attached nodules, while the influence of nodule size was minimal, Wang concluded.
Wang said in response to a question that automated segmentation is poor for nodules attached to vessel walls in the current software version, so these nodules must be evaluated manually.
Measuring the solid part of GGOs
In a third ECR presentation, Ph.D. student Jing Huo from the David Geffen School of Medicine at the University of California, Los Angeles (UCLA) presented a study using an automated solid component detection method. The process was developed to evaluate the solid component of ground-glass nodules containing both solid and nonsolid components from presegmented ground-glass opacities (GGOs).
Ground-glass nodules (GGNs) can be classified as pure GGNs, containing only nonsolid (low-density) elements, and mixed GGNs, containing both nonsolid and solid components. Recent research shows that mixed GGNs are more likely to be malignant, and that the ratio of solid component over the entire GGN is an important index for nodule characterization.
"High-intensity structures within the volume of interest are first segmented by an automatically determined optimal threshold; then shape analysis is applied to remove any vessel that is erroneously included by the threshold-based segmentation," the authors from UCLA and New Jersey's Princeton University explained in their abstract.
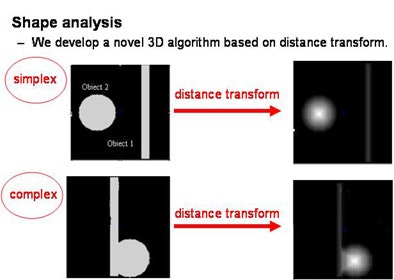
"We use adaptive thresholding, which is based on histogram to find the optimal threshold between pure ground-glass part and the solid component," Huo explained in an e-mail to AuntMinnie.com. "In shape analysis, we developed a 3D algorithm based on distance transform. We classify objects into either simplex (either a solid component or a vessel) or complex (solid components attached to a vessel). We use the algorithm to differentiate them, and for each case we use different tricks to cut the vessel segments."
The method was tested on 16 mixed GGNs. CT volumes were acquired by multislice CT scanners using 1-mm slice collimation.
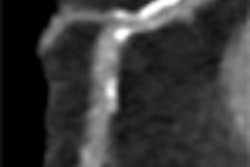
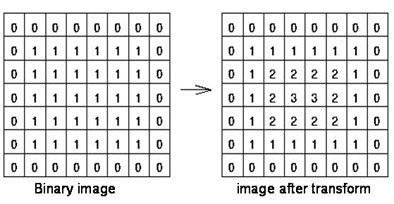 |
| A 3D algorithm based on the theory of distance transform (above) is used to classify objects into either simplex (a solid component or vessel) or complex (solid components attached to a vessel) (below). This algorithm is used to differentiate the objects. All images courtesy of Jing Huo. |
 |
At this point the algorithm is applied to "predefined ground-glass nodules, so we compare the CT numbers of solid part to the pure ground-glass part. But we don't consider the tissues outside of the predefined contour," he added.
"Nodule size varied from 20 mm to 150 mm in the 16 nodules, resolution was 1 mm for the z axis, and 0.49 to 0.78 mm for x-y resolution," Huo said. This enabled visualization of vessels approximately 100 mm or larger.
Lacking a quantitative method of assessing the method, the researchers defined three satisfaction levels for the segmentation result: good, acceptable, or failed.
In all, 75% of the solid components were correctly segmented with the level good or acceptable, Huo said (n = 7 good, n = 5 acceptable, n = 4 failed).
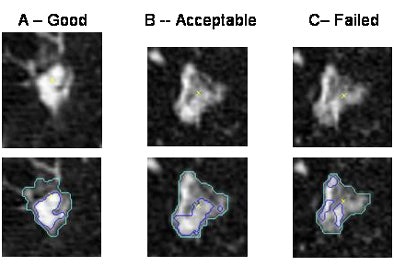 |
| Examples of good (left), acceptable (middle), and failed results. |
The method offers fast and reproducible measures of the ratio of solid components within GGNs for computer-aided diagnosis, extending the performance of CAD systems, Huo concluded.
By Eric Barnes
AuntMinnie.com staff writer
April 16, 2007
Related Reading
PET/CT moves to prime time as first-line tool for lung cancer diagnosis, March 10, 2007
CAD performs well in lung nodule detection, February 5, 2007
CAD turns in mixed performance for pulmonary embolism, January 16, 2007
PET, CT CAD scheme differentiates benign from malignant lung nodules, November 28, 2006
Lung CT CAD boosts performance of less experienced radiologists, November 27, 2006
Copyright © 2007 AuntMinnie.com