Neusoft Medical Systems of Shenyang, China, has received U.S. Food and Drug Administration (FDA) clearance for its NeuViz 16 multislice CT scanner.
NeuViz 16 is designed with an integrated detector for enhanced signal-to-noise ratio and scanning time. The company's patented dynamic focal spot technology offers a greater spatial resolution during scanning and creates enhanced 3D multiplanar reformatted images, according to Neusoft.
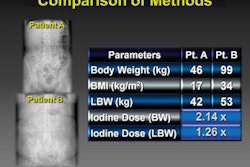
The scanner also features the company's DoseRight modulation technique and a pediatric protocol for dose optimization.
Related Reading
Neusoft Positron files 510(k) for Attrius, January 29, 2009
Neusoft taps TUV for FDA help, October 23, 2007
Positron opens Chinese joint venture, February 13, 2006
Copyright © 2009 AuntMinnie.com